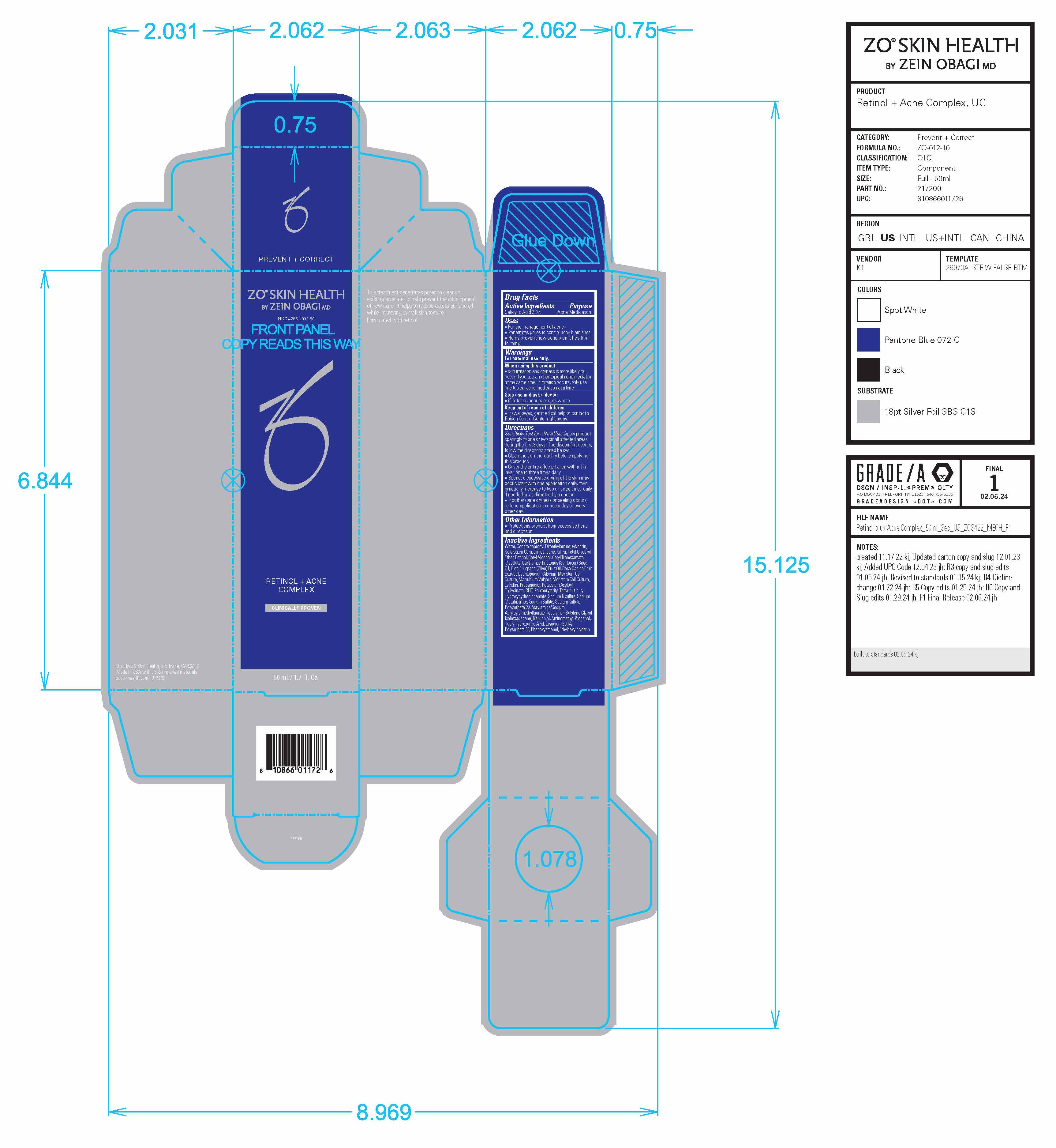
Label: ZO SKIN HEALTH RETINOL PLUS ACNE COMPLEX- salicylic acid lotion
- NDC Code(s): 42851-093-50
- Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Uses
- For the management of acne.
- Penetrates pores to control acne blemishes.
- Helps prevent new acne blemishes from forming.
Warnings
Directions
Sensitivity Test for a New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated below.
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
Inactive Ingredients
Water, Cocamidopropyl Dimethylamine, Glycerin,
Sclerotium Gum, Dimethicone, Silica, Cetyl Glyceryl
Ether, Retinol, Cetyl Alcohol, Cetyl Tranexamate
Mesylate, Carthamus Tinctorius (Safflower) Seed
Oil, Olea Europaea (Olive) Fruit Oil, Rosa Canina Fruit
Extract, Leontopodium Alpinum Meristem Cell
Culture, Marrubium Vulgare Meristem Cell Culture,
Lecithin, Propanediol, Potassium Azeloyl
Diglycinate, BHT, Pentaerythrityl Tetra-di-t-butyl
Hydroxyhydrocinnamate, Sodium Bisulfite, Sodium
Metabisulfite, Sodium Sulfite, Sodium Sulfate,
Polysorbate 20, Acrylamide/Sodium
Acryloyldimethyltaurate Copolymer, Butylene Glycol,
Isohexadecane, Bakuchiol, Aminomethyl Propanol,
Caprylhydroxamic Acid, Disodium EDTA,
Polysorbate 80, Phenoxyethanol, Ethylhexylglycerin. - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH RETINOL PLUS ACNE COMPLEX
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-093 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 mg in 50 mL Inactive Ingredients Ingredient Name Strength AMINOMETHYLPROPANOL (UNII: LU49E6626Q) BAKUCHIOL (UNII: OT12HJU3AR) COCAMIDOPROPYL DIMETHYLAMINE (UNII: L36BM7DG2T) RETINOL (UNII: G2SH0XKK91) OLIVE OIL (UNII: 6UYK2W1W1E) POTASSIUM AZELOYL DIGLYCINATE (UNII: N02RVN6NYP) SODIUM BISULFITE (UNII: TZX5469Z6I) ISOHEXADECANE (UNII: 918X1OUF1E) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) SODIUM SULFATE (UNII: 0YPR65R21J) CETYL GLYCERYL ETHER (UNII: P9FNL3D0MN) PROPANEDIOL (UNII: 5965N8W85T) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) WATER (UNII: 059QF0KO0R) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BETASIZOFIRAN (UNII: 2X51AD1X3T) SODIUM METABISULFITE (UNII: 4VON5FNS3C) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM SULFITE (UNII: VTK01UQK3G) SAFFLOWER SEED (UNII: 8FCL6A31TX) CETYL ALCOHOL (UNII: 936JST6JCN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-093-50 1 in 1 CARTON 09/12/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/12/2024 Labeler - ZO Skin Health, Inc. (826468527)