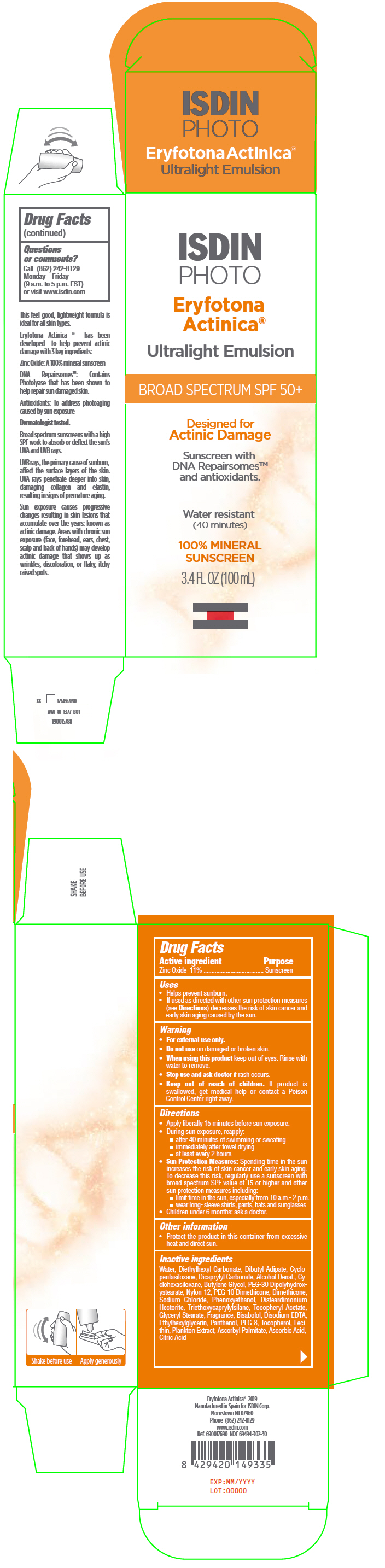
Label: ERYFOTONA ACTINICA ULTRALIGHT EMULSION- sunscreen emulsion
-
NDC Code(s):
69494-302-10,
69494-302-11,
69494-302-12,
69494-302-13, view more69494-302-14, 69494-302-30
- Packager: ISDIN Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warning
-
Directions
- Apply liberally 15 minutes before sun exposure
- During sun exposure, reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures: Spending time in the sun increases the risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.- 2 p.m.
- wear long- sleeve shirts, pants, hats and sunglasses
- Children under 6 months: ask a doctor.
- Other information
-
Inactive ingredients
Water, Diethylhexyl Carbonate, Dibutyl Adipate, Cyclopentasiloxane, Dicaprylyl Carbonate, Alcohol Denat., Cyclohexasiloxane, Butylene Glycol, PEG-30 Dipolyhydroxystearate, Nylon-12, PEG-10 Dimethicone, Dimethicone, Sodium Chloride, Phenoxyethanol, Disteardimonium Hectorite, Triethoxycaprylylsilane, Tocopheryl Acetate, Glyceryl Stearate, Fragrance, Bisabolol, Disodium EDTA, Ethylhexylglycerin, Panthenol, PEG-8, Tocopherol, Lecithin, Plankton Extract, Ascorbyl Palmitate, Ascorbic Acid, Citric Acid.
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 100 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
ERYFOTONA ACTINICA ULTRALIGHT EMULSION
sunscreen emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69494-302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 11 g in 100 mL Inactive Ingredients Ingredient Name Strength LECITHIN, SOYBEAN (UNII: 1DI56QDM62) WATER (UNII: 059QF0KO0R) DIETHYLHEXYL CARBONATE (UNII: YCD50O0Z6L) Dibutyl Adipate (UNII: F4K100DXP3) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) Dicaprylyl Carbonate (UNII: 609A3V1SUA) Alcohol (UNII: 3K9958V90M) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) CYCLOMETHICONE 6 (UNII: XHK3U310BA) Butylene Glycol (UNII: 3XUS85K0RA) PEG-30 DIPOLYHYDROXYSTEARATE (4000 MW) (UNII: 9713Q0S7FO) Nylon-12 (UNII: 446U8J075B) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) Dimethicone (UNII: 92RU3N3Y1O) Sodium Chloride (UNII: 451W47IQ8X) Phenoxyethanol (UNII: HIE492ZZ3T) Disteardimonium Hectorite (UNII: X687XDK09L) Triethoxycaprylylsilane (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LEVOMENOL (UNII: 24WE03BX2T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Ethylhexylglycerin (UNII: 147D247K3P) Panthenol (UNII: WV9CM0O67Z) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Tocopherol (UNII: R0ZB2556P8) Ascorbyl Palmitate (UNII: QN83US2B0N) Ascorbic Acid (UNII: PQ6CK8PD0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) ANACYSTIS NIDULANS (UNII: UV4FTL6UAW) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69494-302-30 1 in 1 CARTON 11/01/2019 1 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:69494-302-10 1 in 1 CARTON 11/01/2019 01/01/2021 2 10 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 3 NDC:69494-302-12 1 in 1 CARTON 11/01/2019 3 2 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 4 NDC:69494-302-13 1 in 1 CARTON 05/01/2023 4 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 5 NDC:69494-302-14 12 in 1 CARTON 10/30/2023 5 4 mL in 1 TUBE; Type 0: Not a Combination Product 6 NDC:69494-302-11 12 in 1 CARTON 12/18/2023 6 2 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 08/17/2015 Labeler - ISDIN Corp (079609155) Registrant - ISDIN Corp (079609155)