Label: POSILAC- sometribove suspension
- NDC Code(s): 86106-0225-1, 86106-0225-2, 86106-0225-3
- Packager: Union Agener Inc
- Category: OTC ANIMAL DRUG LABEL
Drug Label Information
Updated January 29, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
- INDICATIONS & USAGE
- SPL UNCLASSIFIED SECTION
- DOSAGE & ADMINISTRATION
- DOSAGE & ADMINISTRATION
-
SPL UNCLASSIFIED SECTION
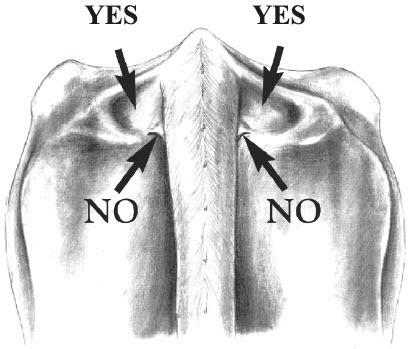
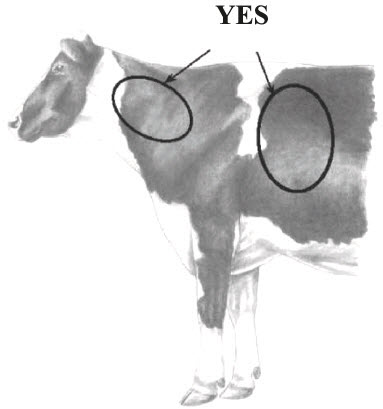
INJECTION TECHNIQUE: Inject Posilac subcutaneously (under the skin). Recommended injection sites are neck area, behind the shoulder or in the depression on either side of the tailhead (see diagrams below). Alternate between the cow's left and right side on consecutive injections. Remove surface dirt from the injection site area before injecting. Inject entire contents of the syringe subcutaneously. Do not reuse syringes.
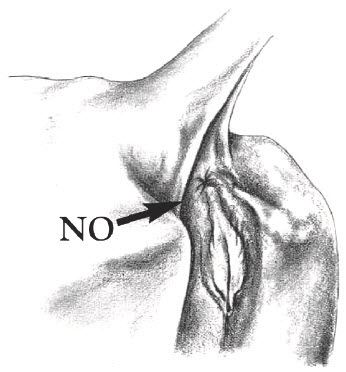
Inject directly into the deepest depressions on either side of the tailhead (marked "Yes"). Avoid the bone, muscles, tendons and ligaments of the tail and the rectal and anal muscles. Do NOT inject into the caudal fold (marked "No") because this may invalidate USDA tuberculosis testing. Locate the caudal fold by raising the tail.
-
WARNINGS
USER SAFETY WARNINGS: Not for use in humans, Keep out of reach of children, Avoid prolonged or repeated contact with Posilac with eyes and skin, Posilac is a protein. Frequent skin contact with proteins may produce an allergic reaction in some people. Always wash hands and skin exposed to Posilac with soap and water after handling. Clothing soiled with the product should be laundered before reuse.
-
ANIMAL SAFETY WARNINGS:
- Use in lactating dairy cows only.
- Safety to replacement bulls born in dairy cows injected with Posilac has not been established.
- Avoid injecting within 2 weeks of slaughter to minimize injection site blemishes on carcass.
- Nutritional Management: Cows injected with Posilac increase voluntary feed intake over several weeks following the start of supplementation. This increase occurs sooner for first lactation cows than for second lactation or older cows. The increased feed intake continues during supplementation and may continue through the dry period and the following early lactation. However, cows treated with Posilac tend to maintain lower body condition than untreated cows. This effect is more pronounced for second lactation or older cows.
- Feed diets formulated to meet or exceed the nutritional requirements recommended by the National Research Council. Consider milk yield, stage of lactation, and body condition when making dietary changes. Manage the feeding program to optimize milk yield and to have cows in appropriate body condition, particularly during late lactation and the dry period. Increasing the energy density of diets fed to cows treated with Posilac is normaly not required. Avoid sudden dietary changes.
- Reproduction: Cows injected with Posilac may have reduced pregnancy rates and increased days open. Have a comprehensive and ongoing herd reproductive health program In place on your dairy before using Posilac.
- Mastitis: Cows injected with Posilac are at an increased risk of mastitis (visibly abnormal milk) and may have higher somatic cell counts. Have comprehensive mastitis management practices in place on your dairy before using Posilac.
- General Health: Cows injected with Posilac may require more therapeutic drug treatment for mastitis and other health problems. Cows injected with Posilac may experience periods of increased body temperature unrelated to illness. To minimize the effect, take appropriate measures during periods of high environmental temperature to reduce heat stress. Use care to differentiate whether increased body temperature is caused by illness or use of Posilac. Cows injected with Posilac may have more enlarged hocks and disorders of the foot region. Posilac treatment may reduce hemoglobin and hematocrit values.
- Injection Site Reactions: A mild temporary swelling of 3-5 cm (1-2 inches) in diameter may occur at the injection site beginning about 3 days after injection and may persist up to 6 weeks following injection. Larger swellings may occur in cows injected in the neck area compared to the behind the shoulder or in the depression on either side of the tailhead. Some cows may experience swellings up to 10 cm (4 inches) in diameter that remain permanent but are not associated with animal health problems. However, if permanent blemishes are objectionable to you, stop supplementation of these cows. Also stop using Posilac in cows with injection site swellings that repeatedly open and drain.
- ADDITIONAL INFORMATION:
- STORAGE AND HANDLING
- ENVIRONMENTAL WARNING
- HOW SUPPLIED
- QUESTIONS/COMMENTS?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 25 Syringe Carton
Posilac (sometribova suspensión de zinc)
Mantener en refrigeración (2 °C a 8 °C; 36° F a 46 °F). NO CONGELAR. Permita que las jeringas alcancen la temperatura ambiente
(15 °C a 30 °C; 59 ° a 86 °F) antes de usar. Evite la exposición prolongada a temperaturas excesivamente altas y a la luz solar.Elaborado por
Union Agener, Inc.
1788 Lovers Lane, Augusta, Georgia 30901, EUA¿PREGUNTAS/COMENTARIOS?
Conctate a Union Agener, Inc. al +1 844-952-0330. Para reportar efectos secundarios, conctate Union Agener, Inc.: +1 844-952-0330. Para obtener informaciones
adicionales sobre cómo reportar efectos secundarios de medicamentos para animales, conctate FDA +1 1-888-FDA-VETS o http://www.fda.gov/reportanimalaeAprobado por la FDA bajo número NADA # 140-872

-
INGREDIENTS AND APPEARANCE
POSILAC
sometribove suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86106-0225 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength sometribove (UNII: PBK5EQG5CQ) (sometribove - UNII:PBK5EQG5CQ) sometribove 500 mg Inactive Ingredients Ingredient Name Strength Sesame Oil (UNII: QX10HYY4QV) Aluminum Monostearate (UNII: P9BC99461E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86106-0225-3 25 in 1 CARTON 1 NDC:86106-0225-1 1 in 1 SYRINGE 2 NDC:86106-0225-2 100 in 1 CARTON 2 NDC:86106-0225-1 1 in 1 SYRINGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA140872 03/01/2020 Labeler - Union Agener Inc (116587901) Establishment Name Address ID/FEI Business Operations Union Agener Inc. 116587901 ANALYSIS, API MANUFACTURE, LABEL, MANUFACTURE, PACK