Label: FENTY SKIN HYDRA VIZOR SPF 30- avobenzone, homosalate, octisalate lotion
- NDC Code(s): 71499-220-01, 71499-220-02, 71499-220-03
- Packager: KENDO HOLDINGS INC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
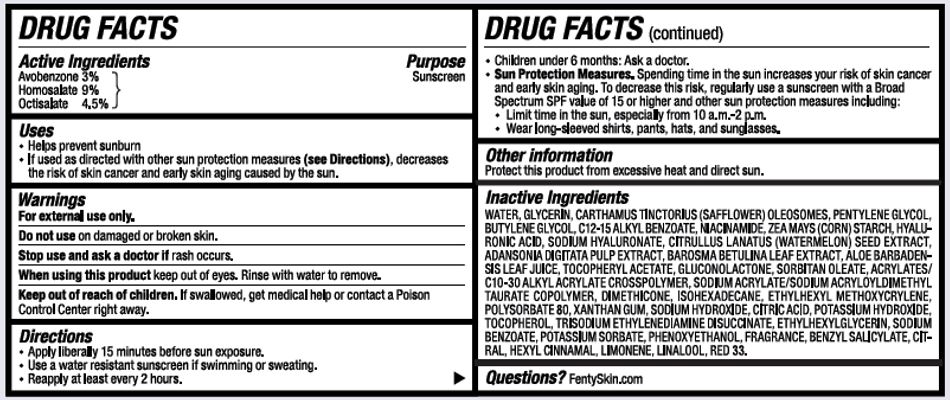
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
- APPLY LIBERALLY 15 MINUTES BEFORE SUN EXPOSURE.
- USE A WATER RESISTANT SUNSCREEN IF SWIMMING OR SWEATING.
- REAPPLY AT LEAST EVERY 2 HOURS.
- CHILDREN UNDER 6 MONTHS: ASK A DOCTOR.
- SUN PROTECTION MEASURES. SPENDING TIME IN THE SUN INCREASES YOUR RISK OF SKIN CANCER AND EARLY SKIN AGING. TO DECREASE THIS RISK, REGULARLY USE A SUNCREEN WITH A BROAD SPECTRUM SPF VALUE OF 15 OR HIGHER AND OTHER SUN PROTECTION MEASURES INCLUDING:
- LIMIT TIME IN THE SUN, ESPECIALLY FROM 10 A.M. - 2 P.M.
- WEAR LONG=SLEEVED SHIRTS, PANTS, HATS, AND SUNGLASSES.
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
WATER, GLYCERIN, CARTHAMUS TINCTORIUS SEED OLEOSOMES, PENTYLENE GLYCOL, BUTYLENE GLYCOL, C12-15 ALKYL BENZOATE, NIACINAMIDE, ZEA MAYS (CORN) STARCH, HYALURONIC ACID, SODIUM HYALURONATE, CITRULLUS LANATUS (WATERMELON) SEED EXTRACT, ADANSONIA DIGITATA PULP EXTRACT, BAROSMA BETULINA LEAF EXTRACT, ALOE BARBADENSIS LEAF JUICE, TOCOPHERYL ACETATE, GLUCONOLACTONE, SORBITAN OLEATE, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER, DIMETHICONE, ISOHEXADECANE, ETHYLHEXYL METHOXYCRYLENE, POLYSORBATE 80, XANTHAN GUM, SODIUM HYDROXIDE, CITRIC ACID, POTASSIUM HYDROXIDE, TOCOPHEROL, TRISODIUM ETHYLENEDIAMINE DISUCCINATE, ETHYLHEXYLGLYCERIN, SODIUM BENZOATE, POTASSIUM SORBATE, PHENOXYETHANOL, FRAGRANCE, BENZYL SALICYLATE, CITRAL, HEXYL CINNAMAL, LIMONENE, LINALOOL, RED 33.
- QUESTIONS?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FENTY SKIN HYDRA VIZOR SPF 30
avobenzone, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71499-220 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ADANSONIA DIGITATA FRUIT PULP (UNII: P65OHK3GHP) HYALURONIC ACID (UNII: S270N0TRQY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) D&C RED NO. 33 (UNII: 9DBA0SBB0L) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TOCOPHEROL (UNII: R0ZB2556P8) BENZYL SALICYLATE (UNII: WAO5MNK9TU) CITRAL (UNII: T7EU0O9VPP) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) PENTYLENE GLYCOL (UNII: 50C1307PZG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) NIACINAMIDE (UNII: 25X51I8RD4) STARCH, CORN (UNII: O8232NY3SJ) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) PHENOXYETHANOL (UNII: HIE492ZZ3T) ISOHEXADECANE (UNII: 918X1OUF1E) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) POLYSORBATE 80 (UNII: 6OZP39ZG8H) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCONOLACTONE (UNII: WQ29KQ9POT) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SODIUM BENZOATE (UNII: OJ245FE5EU) AGATHOSMA BETULINA LEAF (UNII: 369DDH39Z0) WATERMELON SEED (UNII: N364973Y9Q) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71499-220-01 7 mL in 1 TUBE; Type 0: Not a Combination Product 08/23/2020 2 NDC:71499-220-02 30 mL in 1 TUBE; Type 0: Not a Combination Product 08/23/2020 3 NDC:71499-220-03 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/23/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/23/2020 Labeler - KENDO HOLDINGS INC (078489982)