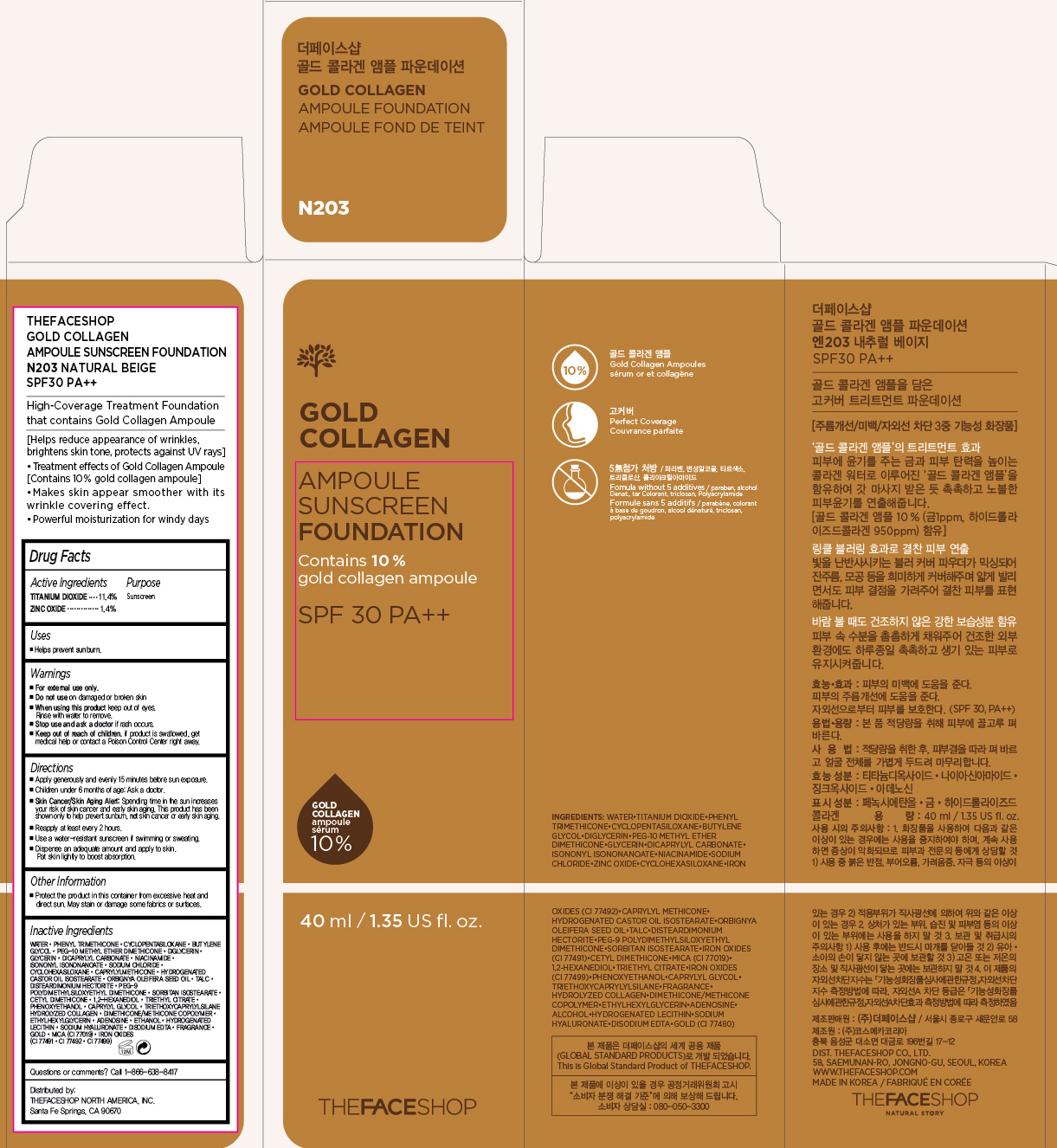
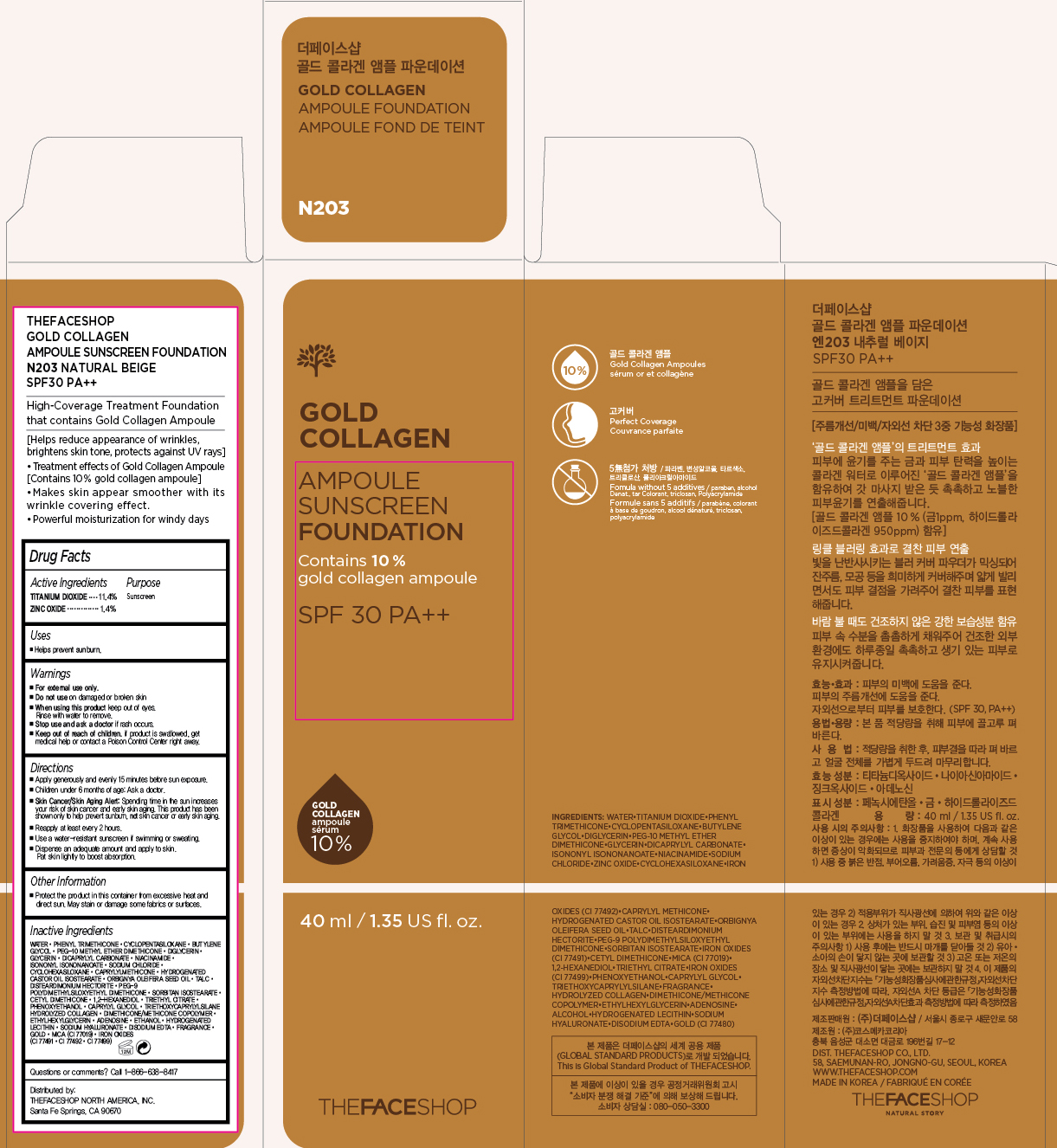
Label: GOLD COLLAGEN AMPOULE FOUNDATION SPF30- titanium dioxide and zinc oxide cream powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 51523-258-09 - Packager: THEFACESHOP CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 31, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Purpose
- Warnings
-
Directions
Apply generously and evenly 15 minutes before sun exposure.
Children under 6 months of age: Ask a doctor.
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
Reapply at least every 2 hours.
Use a water-resistant sunscreen if swimming or sweating.Dispense an adequate amount and apply to skin. Pat skin lightly to boost absorption.
- Other Information
-
Inactive Ingredients
Water, Phenyl Trimethicone, Cyclopentasiloxane, Butylene Glycol, PEG-10 Methyl Ether Dimethicone, Diglycerin, Glycerin, Dicaprylyl Carbonate, Niacinamide, Isononyl Isononanoate, Sodium Chloride, Cyclohexasiloxane, Caprylyl Methicone, Hydrogenated Castor Oil Isostearate, Orbignya Oleifera Seed Oil, Talc, Disteardimonium Hectorite, PEG-9 Polydimethylsiloxyethyl Dimethicone, Sorbitan Isostearate, Cetyl Dimethicone, 1,2-Hexanediol, Triethyl Citrate, Phenoxyethanol, Caprylyl Glycol, Triethoxycaprylylsilane Hydrolyzed Collagen, Dimethicone/Methicone Copolymer, Ethylhexylglycerin, Adenosine, Ethanol, Hydrogenated Lecithin, Sodium Hyaluronate, Disodium EDTA, Fragrance, Gold, Mica (CI 77019), Iron Oxides (CI 77491, CI 77492, CI 77499)
- Questions
- Distributed by:
-
PRINCIPAL DISPLAY PANEL
THEFACESHOP GOLD COLLAGEN
AMPOULE SUNSCREEN FOUNDATION
N203 NATURAL BEIGE SPF30 PA+++
---------------------------------------------------
High-Coverage Treatment Foundation that contains Gold Collagen Ampoule
----------------------------------------------------
[Helps reduce appearance of wrinkles, brightens skin tone, protects against UV rays]
- Treatment effects of Gold Collangen Ampoule [Contains 10% gold collagen ampoule]
- Makes skin appear smoother with its wrinkle covering effect
- Powerful moisturization for windy days
-
INGREDIENTS AND APPEARANCE
GOLD COLLAGEN AMPOULE FOUNDATION SPF30
titanium dioxide and zinc oxide cream powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51523-258 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.6 g in 40 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.6 g in 40 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51523-258-09 1 in 1 CARTON 12/31/2015 1 40 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/31/2015 Labeler - THEFACESHOP CO., LTD. (688329416) Registrant - THEFACESHOP NORTH AMERICA, INC. (620459193) Establishment Name Address ID/FEI Business Operations THEFACESHOP CO., LTD. 688329416 label(51523-258) Establishment Name Address ID/FEI Business Operations Cosmecca Korea Co.,Ltd. 688830827 manufacture(51523-258)