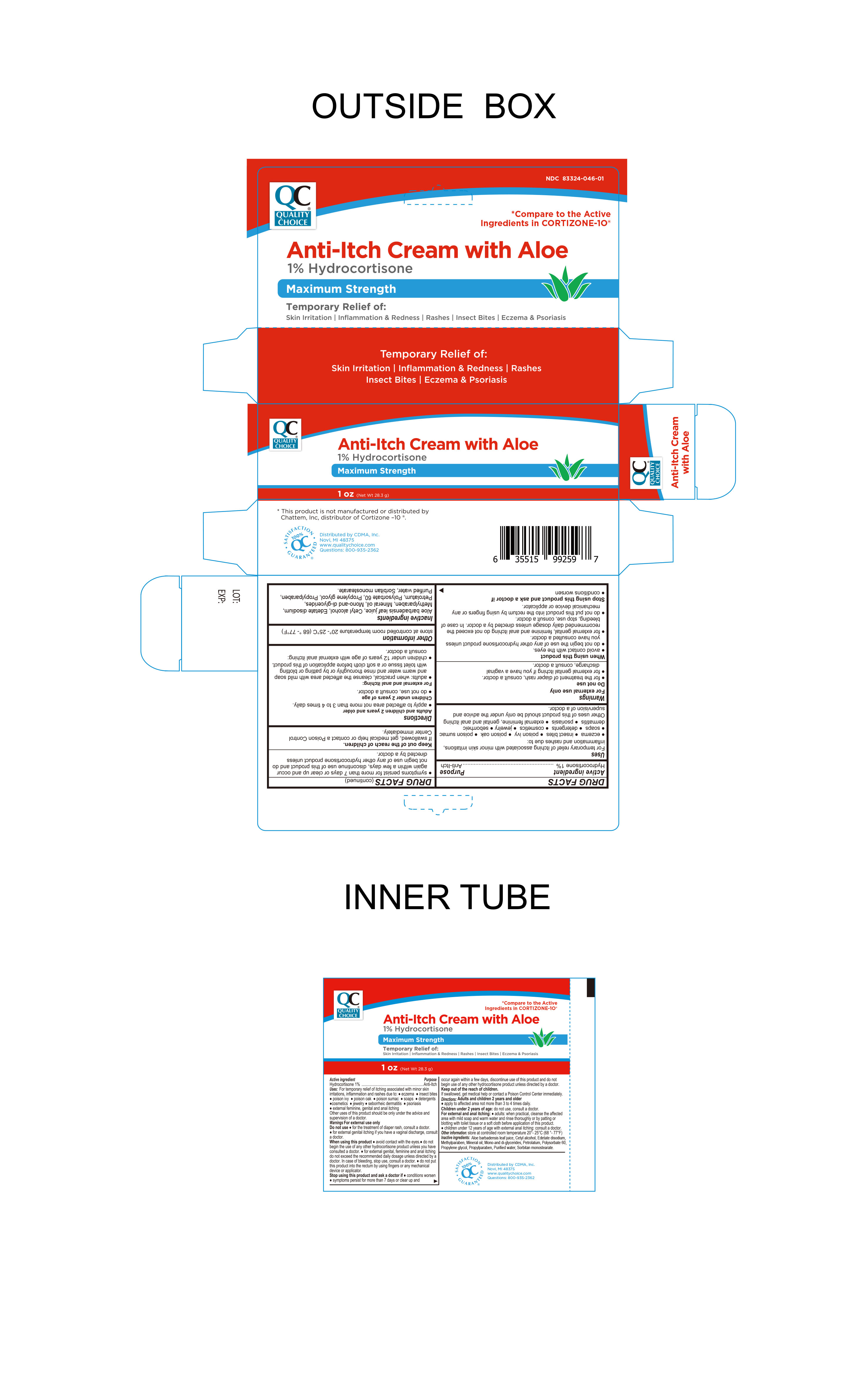
Label: ANTI-ITCH WITH ALOE- hydrocortisone 1% cream
- NDC Code(s): 83324-046-01
- Packager: Chain Drug Marketing Association Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
INDICATIONS & USAGE
For the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to
- insect bites
- eczema
- poison ivy
- poison oak
- poison sumac
- soaps
- detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- external feminine genital and anal itching
- Other uses of this product should be only under the advice and supervision of a doctor.
- Warnings
- Stop use and ask a doctor
- Keep out of the reach of children
- Other information
- Inactive Ingredients
-
When using this product
- Avoid contact with the eyes
- do not begin the use of any other hydrocortisone product unless you have consulted a doctor.
- for external genital, feminine and anal itching do not exceed the recommended daily dosage unless directed by a doctor. In the case of bleeding, stop use, consult a doctor.
- do not put this product into the rectum by using fingers or any mechanical device or applicator.
-
Directions
Adults and children 2 years and older
- apply to affected area not more than 3 to 4 times daily.
Children under 2 years of age
- do not use, consult doctor
For external and anal itching
- adults when practical cleanse the affected area with mild soap and warm water and rinse thoroughly or by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Children under 12 years of age with external anal ithcing consult a doctor.
- Distributed By
- Packaging
-
INGREDIENTS AND APPEARANCE
ANTI-ITCH WITH ALOE
hydrocortisone 1% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83324-046 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) ALOE VERA LEAF (UNII: ZY81Z83H0X) EDETATE DISODIUM (UNII: 7FLD91C86K) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) PETROLATUM (UNII: 4T6H12BN9U) MINERAL OIL (UNII: T5L8T28FGP) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERYL MONO AND DIPALMITOSTEARATE (UNII: KC98RO82HJ) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83324-046-01 1 in 1 BOX 06/13/2024 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 06/13/2024 Labeler - Chain Drug Marketing Association Inc. (011920774) Registrant - Trifecta Pharmaceuticals USA, LLC. (079424163)