Label: CARE SCIENCE OSHA FIRST AID- ethyl alcohol, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin-b sulfate, diphenhydramine hydrochloride, hydrocortisone, ibuprofen, acetaminophen, water kit
-
NDC Code(s):
47682-167-46,
47682-198-28,
47682-718-99,
47682-803-99, view more51142-002-01, 51142-445-21, 61010-1112-1, 61010-2017-0, 61010-5600-1, 61010-5800-1
- Packager: ASO LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
CONTENTS – Care Science OSHA First Aid Kit
Clean 68 ITEMS
39 Alcohol Prep Pads 1 3/16 IN X 2 5/16 IN (30 MM X 58 MM)12 Antiseptic Wipes 4 3/4 IN X 7 3/4 IN (120 MM X 196 MM)
10 Hand Sanitizers 1/32 OZ (0.9 G)
6 Hand Cleansing Wipes 4 3/4 IN X 7 3/4 IN (120 MM X 196 MM)
1 Eye Wash 1 FL OZ (30 ML)
Treat 40 ITEMS
14 Triple Antibiotic Ointments 1/32 OZ (0.9 G)
10 Topical Cooling Gel 1/8 OZ (3.5 G)
1 Acetaminophen (Non-aspirin) 2 Per Packet
1 Diphen Allergy Caplet 1 Per Packet
10 Hydrocortisone Cream 1/32 OZ (0.9 G)
2 Ibuprofen 2 Per Packet
1 Burn Dressing 4 IN X 4 IN (101 MM X 101 MM)
1 Instant Cold Pack, Single Use 4 IN X 5 IN (101 MM X 125 MM)
Protect 226 ITEMS
70 Sheer Bandages 3/4 IN X 3 IN (19 MM X 76 MM)
40 Sheer Bandages 1 IN X 3 IN (25 MM X 76 MM)
8 Gauze Pads 2 IN x 2 IN (50 MM x 50 MM)
4 Transparent Dressings 2 3/8 IN X 4 IN (60 MM X 101 MM)
2 Eye Cover Pads 2.25 IN x 3.03 IN (57 MM x 77 MM)
60 Sheer Bandages 3/8 IN X 1 1/2 IN (9.5 MM X 38 MM)
35 Butterfly Closures 1 3/4 IN X 3/8 IN (44 MM X 9.5 MM)
4 Gauze Pads 4 IN x 4 IN (101 MM x 101 MM)
2 Trauma Pads 5 in x 9 in (127 MM x 228 MM)
1 Rolled Gauze 4 IN x 2 1/2 YDS (101 MM x 2.2 M)
Instruments 17 ITEMS
7 Wooden Finger Splints
1 CPR Breathing Barrier
1 Triangular Sling 40 IN X 40 IN X 56 IN (101 CM X 101 CM X 142 CM)
1 Metal Tweezers
4 Nitrile Exam Gloves Non-sterile, single use only
1 Paper Tape 1 IN X 5 YDS (25 MM X 4.5 M)
1 Metal Scissors
1 First Aid Guide
Adhesive Bandages, Burn Dressing, Butterfly Closures, Eye Cover Pad, Gauze Pads, Rolled Gauze, Transparent Dressings, & Trauma Pads are sterile unless wrapper is opened or damaged.
Gauze Pads and Rolled Gauze are Rayon-polyester blend.Kit contents are not made with natural rubber latex.
Alcohol Prep Pads, Eye Wash, Acetaminophen (Non-Aspirin), Diphen Allergy Caplets, Hydrocortisone Cream, Ibuprofen, & Triple Antibiotic Ointment
Caution: This product contains non-prescription drug products that have expiration dates. Please check before use. Keep these and all drug products out of the reach of children. Tamper evident sealed packets; do not use any opened or torn packets.
Adhesive Bandages: For use on minor cuts, scrapes, & burns.Directions: For optimal results, apply bandage to clean, dry skin. Change the dressing daily, when wet, or more often if needed. Single use.
Warning: For medical emergencies, seek professional help.
Butterfly Closures: For use on minor cuts, scrapes, & burns.
Directions: Remove backing tabs from Butterfly Closure. Apply adhesive sections on either side of the wound. Be sure to center the nonstick area over the wound. Change dressing as directed by your health care professional.
Warning: For medical emergencies, seek professional help.
Topical Cooling Gel: For minor burns and scalds
Directions: Apply gel liberally without rubbing it in. Do not use more than 4 times a day. For children under 2 years consult a physician.
Warning: For external use only. Keep out of eyes. Keep out of reach of children. Use only as directed. If allergic reaction occurs, discontinue use and consult a physician.
Caution: Not for serious burns. If the condition for which this product is used persists for more than 7 days, or irritation, redness, swelling or pain increases, or a rash or infection develops, discontinue use and consult a physician. Do not use packet if opened or torn.
Gauze Pads: For cleaning wounds.Directions: Gently clean the wound with mild soap and water using the gauze pad and carefully dry the affected area. Discard the used pad.
Warning: In case of deep puncture wounds or serious burns, consult a physician.
Nitrile Exam Gloves: Non-sterile single use disposable exam gloves.
Storage: Protect from freezing. Avoid excessive heat. Keep dry. Gloves should be shielded from direct sunlight, fluorescent lighting, x-rays, moisture and Ozone.
Disposal: Dispose of gloves and all biologically contaminated matter in an appropriate container.
Paper Tape: For securing wound covers.
Directions: Use gauze to gently clean in and around injured area with mild soap and water. Dry injured area and apply medication if necessary. Cover wound with non-stick pad or dressing. Secure the dressing with paper tape to help keep out dirt and contaminants.
Warning: In case of deep puncture wounds or serious burns, consult a physician.
Rolled Gauze: For securing wound covers.
Directions: Use gauze to gently clean in and around injured area with mild soap and water. Dry injured area and apply medication if necessary. Cover wound with a non-stick pad or dressing. Secure the dressing in place by loosely wrapping the area with rolled gauze over the top of the dressing. Secure the end of the rolled gauze with tape as needed, being careful not to restrict circulation by wrapping or taping too tightly.
Warning: In case of deep puncture wounds or serious burns, consult a physician.
Trauma Pads: For providing extra cushioning and absorption on wound dressings.
Directions: Carefully cover the wound with a primary, non-stick pad. Place the sterile trauma pad over it for extra absorbency protection. Pad should have the blue line facing up.
Warning: In case of deep puncture wounds or serious burns, consult a physician.
Transparent Dressings: Ideal for minor abrasions, cuts, burns, blisters, scrapes, & post-surgical incisions.
Directions: Gently clean the wound using a gauze pad with mild soap and water. Carefully dry the affected area and apply medication if needed. Cover the wound with the Transparent Dressing. Do not use as a primary dressing on moderately to heavily draining wounds. Do not stretch while applying.
Warning: For any change in wound condition, signs of infection or if you have serious burns or a deep puncture wound seek immediate professional medical attention.
Made in USA with globally sourced materials: Antiseptic Wipes, Butterfly Closures, First Aid Guide, Hand Cleansing Wipes, Hand Sanitizers, Hydrocortisone Cream, Instant Cold Pack, Sheer Adhesive Bandages, Topical Cooling Gel, Triple Antibiotic Ointment, Wooden Finger Splints.
Made in China: Acetaminophen (Non-Aspirin), Alcohol Prep Pads, Breathing Barrier, Burn Dressing, Carrying Case, Diphen Allergy Caplet, Eye Cover Pads, Gauze Pads, Metal Scissors, Metal Tweezers, Nitrile Gloves, Paper Tape, Rolled Gauze, Transparent Dressing, Trauma Pads, Triangular Sling.
Made in India: Ibuprofen.Made in Canada: Eye Wash.
ANSI/ISEA Z308.1-2015, Class A, Type I or II. This kit meets the ANSI/ISEA Z308.1-2015 standard as sold. It contains first aid products which meet performance specifications detailed in the standard at the
below required minimum fill. It will continue to be compliant only when maintained with products that
meet the standard at specified quantities.
REQUIRED MINIMUM FILL16 Adhesive Bandages - 1 IN X 3 IN
1 Adhesive Tape - 2 1/2 YDS Total
10 Antibiotic Application - 1/57 OZ
10 Antiseptic - 1/57 OZ
1 Breathing Barrier
1 Burn Dressing (Gel Soaked) - 4 IN X 4 IN
10 Burn Treatment - 1/32 OZ
1 Cold Pack - 4 IN X 5 IN
2 Eye Covering With Means Of Attachment - 2.9 SQ IN
1 Eye/skin Wash - 1 FL OZ Total
1 First Aid Guide
6 Hand Sanitizer - 1/32 OZ
2 Pair Medical Exam Gloves
1 Roller Bandage - 2 IN X 4 YDS
1 Scissors
2 Sterile Pads - 3 IN X 3 IN
2 Trauma Pad - 5 IN X 9 IN
1 Triangular Bandage - 40 IN X 40 IN X 56 IN
The described kit may be suitable for some businesses. However, the adequacy of the contents for hazards of each work environment should always be evaluated by competent personnel. Kits should be inspected frequently to ensure the completeness and usability of all first aid supplies. Any supply beyond its marked expiration date should be discarded and replaced. For a variety of operations, employers may find that additional first aid supplies and kits are needed.PEEL HERE FOR DRUG FACTS & WARNINGS
- Active ingredient (in each caplet) – Diphen Allergy Caplet
- Purpose – Diphen Allergy Caplet
- Uses – Diphen Allergy Caplet
-
Warnings – Diphen Allergy Caplet
Do not use – Diphen Allergy Caplet
- •
- to make a child sleepy
- •
- with any other product containing diphenhydramine, even one that is used on skin.
Ask a doctor before use if you have – Diphen Allergy Caplet
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to an enlarged prostate gland
- •
- glaucoma
Ask a doctor or pharmacist before use if you are – Diphen Allergy Caplet
- •
- taking sedatives or tranquilizers
- Directions – Diphen Allergy Caplet
- Other information – Diphen Allergy Caplet
- Inactive Ingredients – Diphen Allergy Caplet
- Questions or comments? – Diphen Allergy Caplet
- Active ingredients – Antiseptic Wipes
- Purpose – Antiseptic Wipes
- Uses – Antiseptic Wipes
- Warnings – Antiseptic Wipes
- Directions – Antiseptic Wipes
- Inactive ingredients – Antiseptic Wipes
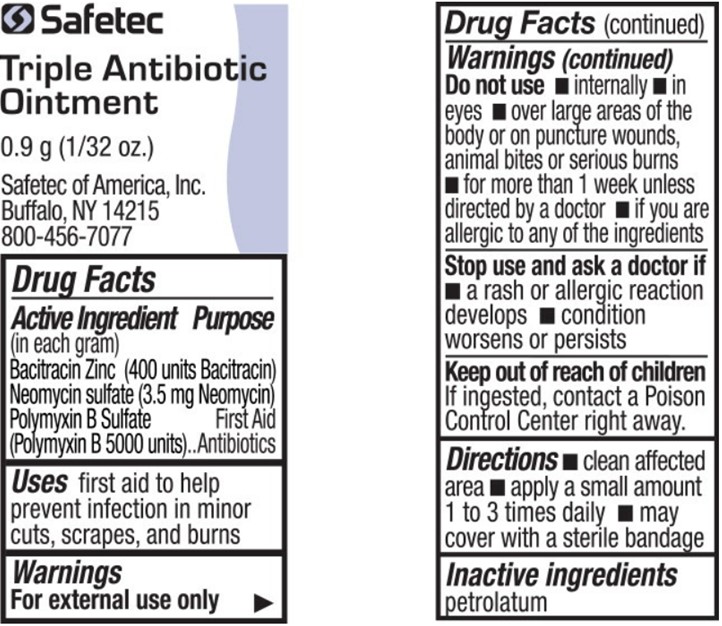
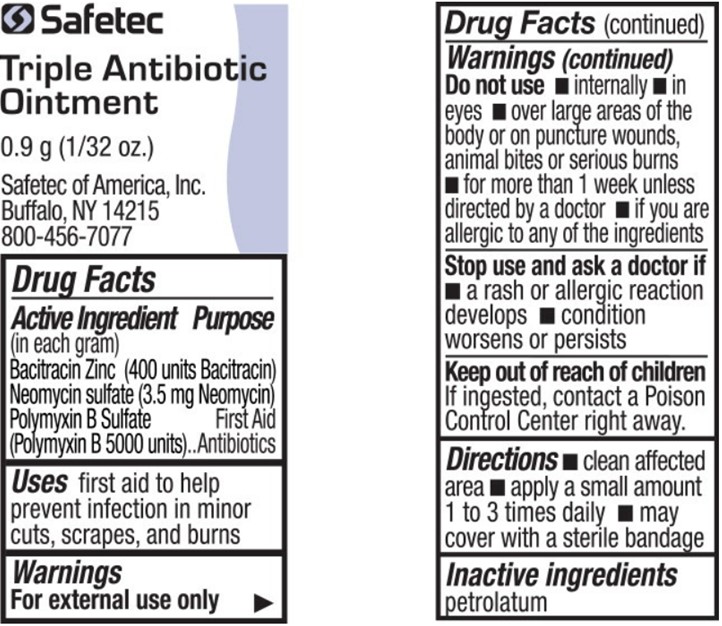
- Active Ingredient (in each gram) – Triple Antibiotic Ointment
- Purpose – Triple Antibiotic Ointment
- Uses – Triple Antibiotic Ointment
-
Warnings – Triple Antibiotic Ointment
For external use only
Do not use – Triple Antibiotic Ointment
- •
- internally
- •
- in eyes
- •
- over large areas of the body or on puncture wounds, animal bites or serious burns
- •
- for more than 1 week unless directed by a doctor
- •
- if you are allergic to any of the ingredients
- Directions – Triple Antibiotic Ointment
- Inactive ingredients – Triple Antibiotic Ointment
- Active Ingredient – Hydrocortisone Cream
- Purpose – Hydrocortisone Cream
- Uses – Hydrocortisone Cream
- Warnings – Hydrocortisone Cream
- Directions – Hydrocortisone Cream
- Inactive ingredients – Hydrocortisone Cream
- Active ingredient – Alcohol Prep Pads
- Purpose – Alcohol Prep Pads
- Uses – Alcohol Prep Pads
-
Warnings – Alcohol Prep Pads
For external use only
Flammable, keep away from fire or flameDo not use – Alcohol Prep Pads
- •
- with electrocautery procedures
- •
- in the eyes. If contact occurs, flush eyes with water.
- Directions – Alcohol Prep Pads
- Other information – Alcohol Prep Pads
- Inactive ingredient – Alcohol Prep Pads
- Active ingredient – Hand Sanitizer
- Purpose – Hand Sanitizer
- Uses – Hand Sanitizer
- Warnings – Hand Sanitizer
- Directions – Hand Sanitizer
- Inactive ingredients – Hand Sanitizer
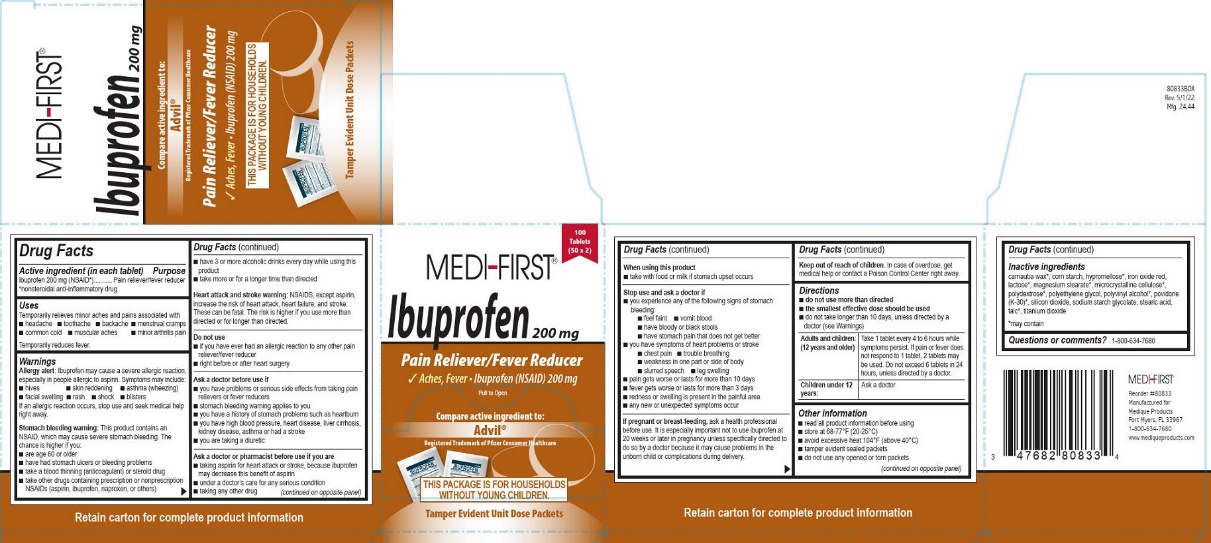
- Active ingredient (in each tablet) – Ibuprofen
- Purpose – Ibuprofen
- Uses – Ibuprofen
-
Warnings – Ibuprofen
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
• hives • skin reddening • asthma (wheezing) • facial swelling • rash • shock • blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
• are age 60 or older
• have had stomach ulcers or bleeding problems
• take a blood thinning (anticoagulant) or steroid drug
• take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
• have 3 or more alcoholic drinks every day while using this product
• take more or for a longer time than directed
Heart attack or stroke warning: NSAIDS, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.Do not use – Ibuprofen
- •
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- •
- right before or after heart surgery
Ask a doctor before use if – Ibuprofen
- •
- you have problems or serious side effects from taking pain relievers or fever reducers
- •
- stomach bleeding warning applies to you
- •
- you have a history of stomach problems such as heartburn
- •
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma or had a stroke
- •
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are – Ibuprofen
- •
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- •
- under a doctor’s care for any serious condition
- •
- taking any other drug
Stop use and ask a doctor if – Ibuprofen
• you experience any of the following signs of stomach bleeding
- •
- feel faint • vomit blood
- •
- have bloody or black stools
- •
- have stomach pain that does not get better
• you have symptoms of heart problems or stroke
- •
- chest pain • trouble breathing
- •
- weakness in one part or side of body
- •
- slurred speech • leg swelling
• pain gets worse or lasts for more than 10 days
• fever gets worse or lasts for more than 3 days
• redness or swelling is present in the painful area
• any new or unexpected symptoms occur -
Directions – Ibuprofen
• do not use more than directed
• the smallest effective dose should be used
• do not take longer than 10 days, unless directed by a doctor (see Warnings)Adults and children: (12 years and older)
Take 1 tablet every 4 to 6 hours while symptoms persist. If pain or fever does not respond to 1 tablet, 2 tablets may be used. Do not exceed 6 tablets in 24 hours, unless directed by a doctor.
Children under 12 years:
Ask a doctor
- Other information – Ibuprofen
- Inactive ingredients – Ibuprofen
- Questions or comments? – Ibuprofen
- Active ingredient (in each tablet) – Acetaminophen
- Purpose – Acetaminophen
- Uses – Acetaminophen
-
Warnings – Acetaminophen
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- •
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: – Acetaminophen
Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use – Acetaminophen
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Ask a doctor or pharmacist before use if – Acetaminophen
- •
- you are taking the blood thinning drug warfarin
-
Directions – Acetaminophen
• do not use more than directed (see overdose warning)
Adults and children: (12 years and over)
- •
- take 2 tablets every 4 to 6 hours while symptoms last
- •
- do not take more than 10 tablets in 24 hours, unless directed by a doctor
- •
- do not use for more than 10 days unless directed by a doctor
Children under 12 years:
- •
- ask a doctor
- Other information – Acetaminophen
- Inactive Ingredients – Acetaminophen
- Questions or comments? – Acetaminophen
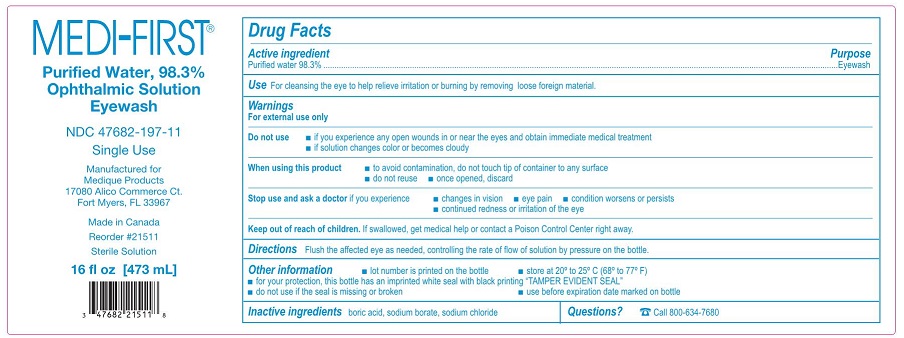
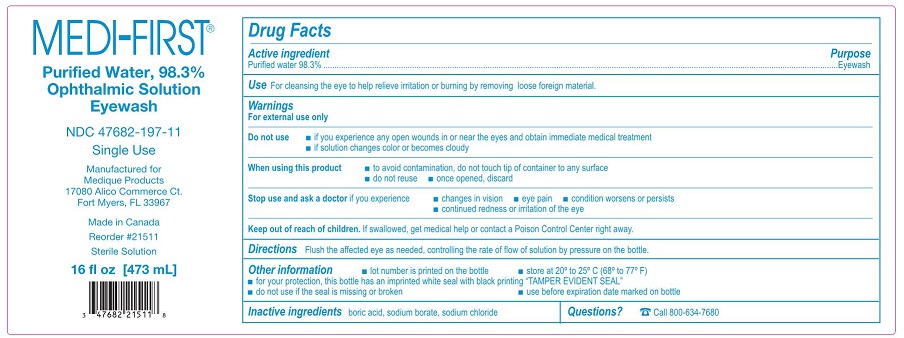
- Active ingredient – Eyewash
- Purpose – Eyewash
- Use – Eyewash
-
Warnings – Eyewash
For external use only
Do not use – Eyewash
- •
- if you experience any open wounds in or near the eyes and obtain immediate medical treatment
- •
- if solution changes color or becomes cloudy
When using this product – Eyewash
- •
- to avoid contamination, do not touch tip of container to any surface
- •
- do not reuse
- •
- once opened, discard
- Directions – Eyewash
- Other information – Eyewash
- Inactive ingredients – Eyewash
- Questions? – Eyewash
-
Principal Display Panel – Care Science OSHA First Aid Kit
CARE SCIENCE®
MEETS OSHA ANSI/ISEA
Z308.1-2015 Guidelines
FIRST AID
OFFICE HOME OUTDOOR SCHOOL
351 PIECES
351 PIECES + 1 CARRYING CASE
See back panel for details- •
- Includes first aid essentials for complete wound care + additional supplies
- •
- Organized shelves for quick access & easy restocking
- •
- Wall mounts for easy access in any setting
ASO
CARE SCIENCE® is an ASO brand
Distributed by ASO LLC
Sarasota, Fl 34240
www.asocorp.comLabel
- Principal Display Panel – Diphen Allergy Caplet
- Principal Display Panel – Antiseptic Wipes
- Principal Display Panel – Triple Antibiotic Ointment
- Principal Display Panel – Hydrocortisone Cream
- Principal Display Panel – Alcohol Prep Pads
- Principal Display Panel – Hand Sanitizer
-
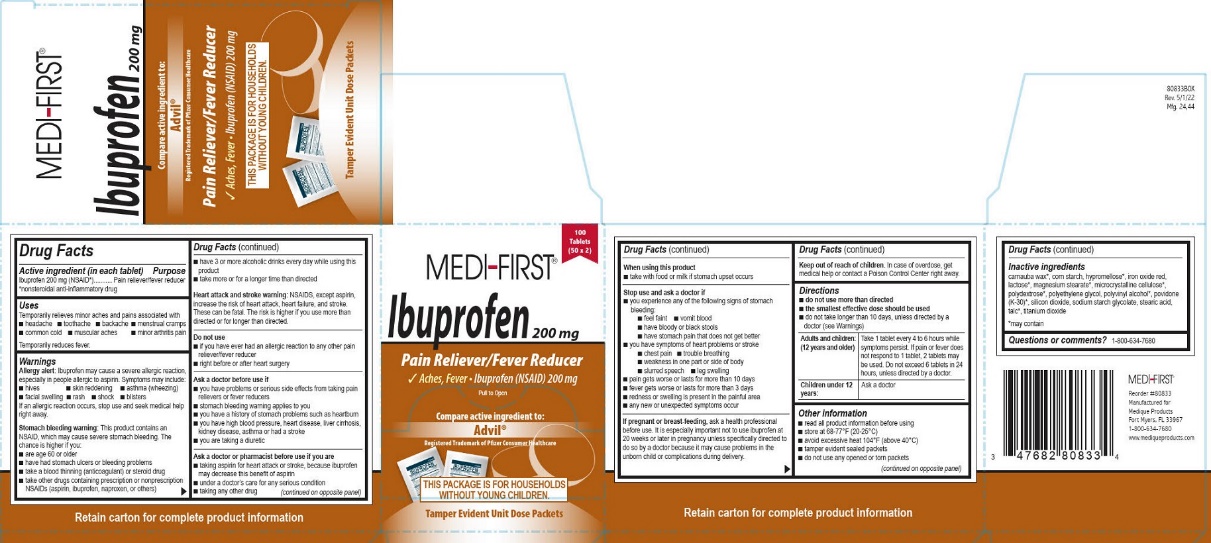
Principal Display Panel – Ibuprofen
MEDI-FIRST®
Ibuprofen 200 mg
100 tablets (50 x 2)
Pain Reliever/Fever Reducer
Aches, Fever • Ibuprofen (NSAID) 200 mg
Pull to Open
Compare active ingredient to:
Advil®
Registered Trademark of Pfizer Consumer Healthcare
THIS PACKAGE IS FOR HOUSEHOLDS
WITHOUT YOUNG CHILDREN.
Tamper Evident Unit Dose Packets
MEDI-FIRST®
Reorder #80833
Manufactured for
Medique Products
Fort Myers, FL 33967
1-800-634-7680
www.mediqueproducts.com
Label
-
Principal Display Panel – Acetaminophen (Non-Aspirin)
Medi-First ®
Non-Aspirin
Aches, Fever • Acetaminophen 325 mg
Compare active ingredient to:
Tylenol®
Registered Trademark of McNeil Consumer products
Pull to Open
Pain Reliever/Fever Reducer
THIS PACKAGE IS FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN.
Tamper Evident Unit Dose Packets
500 Tablets
(250 x 2)Label
- Principal Display Panel – Eye Wash
-
INGREDIENTS AND APPEARANCE
CARE SCIENCE OSHA FIRST AID
ethyl alcohol, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin-b sulfate, diphenhydramine hydrochloride, hydrocortisone, ibuprofen, acetaminophen, water kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51142-002 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51142-002-01 1 in 1 CASE; Type 0: Not a Combination Product 07/20/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PACKET 1 Part 2 12 PACKET 0.0228 L Part 3 14 POUCH 12.6 g Part 4 10 POUCH 9 g Part 5 39 POUCH 13.26 g Part 6 10 POUCH 0.34 L Part 7 2 PACKET 4 Part 8 1 PACKET 2 Part 9 1 BOTTLE, DISPENSING 30 mL Part 1 of 9 MEDIQUE DIPHEN
diphenhydramine hydrochloride tablet, film coatedProduct Information Item Code (Source) NDC:47682-167 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK (pink) Score no score Shape OVAL (OVAL) Size 11mm Flavor Imprint Code 048;D Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-167-46 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/01/2012 Part 2 of 9 ANTISEPTIC
alcohol clothProduct Information Item Code (Source) NDC:61010-2017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 665 mL in 1 L Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-2017-0 0.0019 L in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 05/01/2017 Part 3 of 9 TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:61010-5600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [USP'U] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-5600-1 0.9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 08/08/2011 Part 4 of 9 HYDROCORTISONE
hydrocortisone creamProduct Information Item Code (Source) NDC:61010-5800 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) PETROLATUM (UNII: 4T6H12BN9U) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYSORBATE 60 (UNII: CAL22UVI4M) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) STEARETH-20 (UNII: L0Q8IK9E08) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-5800-1 0.9 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/15/2010 Part 5 of 9 ALCOHOL PREP PAD
alcohol prep pad swabProduct Information Item Code (Source) NDC:51142-445 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (white pad) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51142-445-21 0.34 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 09/24/2018 Part 6 of 9 INSTANT HAND SANITIZER
alcohol gelProduct Information Item Code (Source) NDC:61010-1112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 665 mL in 1 L Inactive Ingredients Ingredient Name Strength ALOE (UNII: V5VD430YW9) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-1112-1 0.034 L in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/15/2010 Part 7 of 9 MEDI-FIRST IBUPROFEN
ibuprofen tablet, coatedProduct Information Item Code (Source) NDC:47682-718 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) POVIDONE K30 (UNII: U725QWY32X) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) FERRIC OXIDE RED (UNII: 1K09F3G675) TALC (UNII: 7SEV7J4R1U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color RED (Reddish Brown) Score no score Shape ROUND Size 10mm Flavor Imprint Code G;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-718-99 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079174 01/26/2017 Part 8 of 9 MEDI-FIRST NON-ASPIRIN
acetaminophen tablet, coatedProduct Information Item Code (Source) NDC:47682-803 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE (WHITE) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code AZ;234 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-803-99 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/30/2008 Part 9 of 9 MEDI-FIRST FIRST AID EYE WASH
purified water solutionProduct Information Item Code (Source) NDC:47682-198 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 983 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-198-28 30 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022305 10/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/20/2023 Labeler - ASO LLC (152793493)