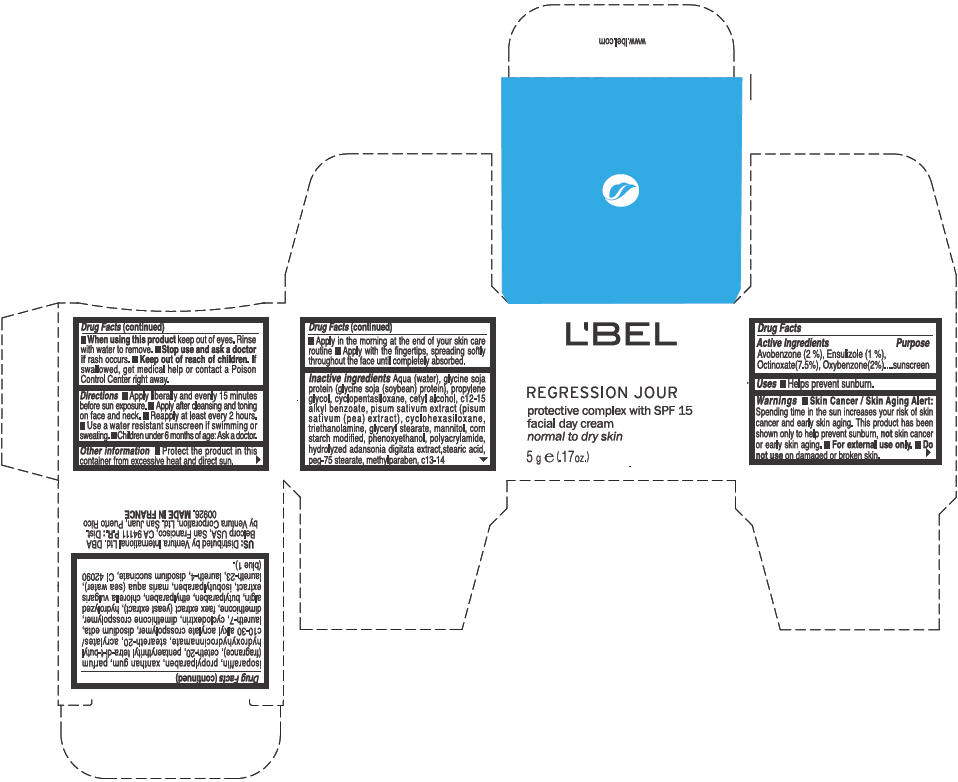
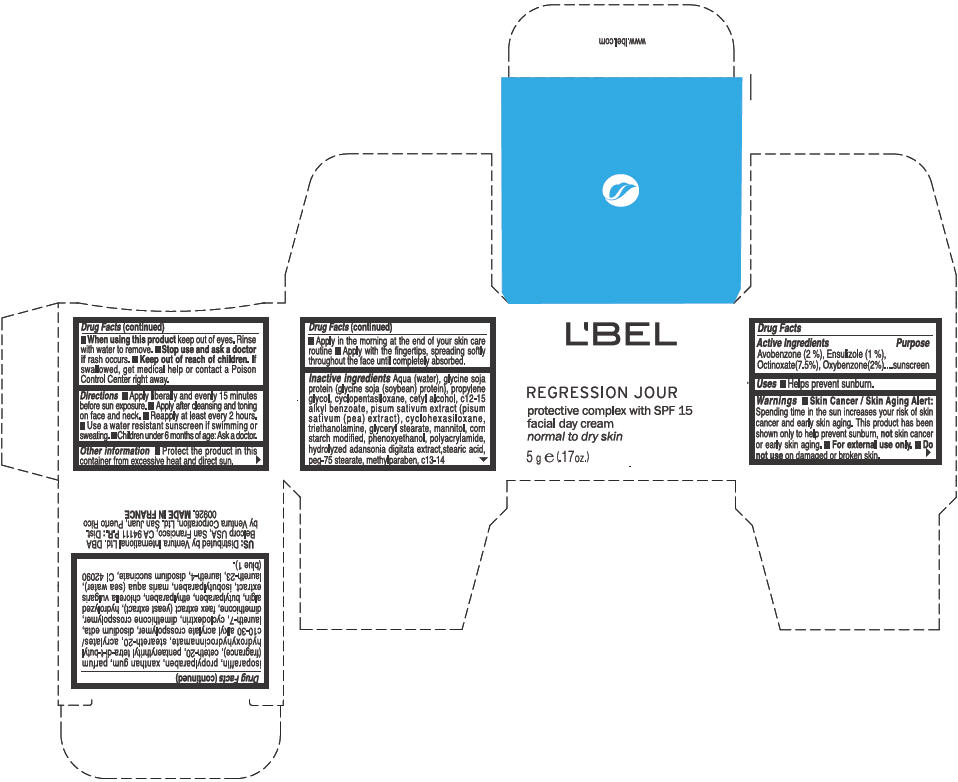
Label: LBEL REGRESSION JOUR PROTECTIVE COMPLEX WITH SPF 15 FACIAL DAY NORMAL TO DRY SKIN- avobenzone, ensulizole, octinoxate, and oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 14783-025-01, 14783-025-03, 14783-025-04, 14783-025-61, view more14783-025-66 - Packager: Ventura International Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 21, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Aqua (water), glycine soja protein (glycine soja (soybean) protein), propylene glycol, cyclopentasiloxane, cetyl alcohol, c12-15 alkyl benzoate, pisum sativum extract (pisum sativum (pea) extract), cyclohexasiloxane, triethanolamine, glyceryl stearate, mannitol, corn starch modified, phenoxyethanol, polyacrylamide, hydrolyzed adansonia digitata extract,stearic acid, peg-75 stearate, methylparaben, c13-14 isoparaffin, propylparaben, xanthan gum, parfum (fragrance), ceteth-20, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, steareth-20, acrylates/c10-30 alkyl acrylate crosspolymer, disodium edta, laureth-7, cyclodextrin, dimethicone crosspolymer, dimethicone, faex extract (yeast extract), hydrolyzed algin, butylparaben, ethylparaben, chlorella vulgaris extract, isobutylparaben, maris aqua (sea water), laureth-23, laureth-4, disodium succinate, CI 42090 (blue 1)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 5 g Jar Box
-
INGREDIENTS AND APPEARANCE
LBEL REGRESSION JOUR PROTECTIVE COMPLEX WITH SPF 15 FACIAL DAY NORMAL TO DRY SKIN
avobenzone, ensulizole, octinoxate, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:14783-025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.02 g in 1 g Ensulizole (UNII: 9YQ9DI1W42) (Ensulizole - UNII:9YQ9DI1W42) Ensulizole 0.01 g in 1 g Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CETYL ALCOHOL (UNII: 936JST6JCN) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) PEA (UNII: W4X7H8GYFM) CYCLOMETHICONE 6 (UNII: XHK3U310BA) TROLAMINE (UNII: 9O3K93S3TK) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MANNITOL (UNII: 3OWL53L36A) PHENOXYETHANOL (UNII: HIE492ZZ3T) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-75 STEARATE (UNII: OT38R0N74H) METHYLPARABEN (UNII: A2I8C7HI9T) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) CETETH-20 (UNII: I835H2IHHX) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) STEARETH-20 (UNII: L0Q8IK9E08) EDETATE DISODIUM (UNII: 7FLD91C86K) LAURETH-7 (UNII: Z95S6G8201) DIMETHICONE (UNII: 92RU3N3Y1O) YEAST (UNII: 3NY3SM6B8U) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) CHLORELLA VULGARIS (UNII: RYQ4R60M02) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) LAURETH-23 (UNII: N72LMW566G) LAURETH-4 (UNII: 6HQ855798J) SODIUM SUCCINATE ANHYDROUS (UNII: V8ZGC8ISR3) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:14783-025-61 1 in 1 BOX 1 NDC:14783-025-66 50 g in 1 JAR 2 NDC:14783-025-04 1 in 1 BOX 2 NDC:14783-025-03 5 g in 1 JAR 3 NDC:14783-025-01 1 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/14/2012 Labeler - Ventura International Ltd. (603192787) Establishment Name Address ID/FEI Business Operations MF Productions Saumur (France) 266769145 MANUFACTURE