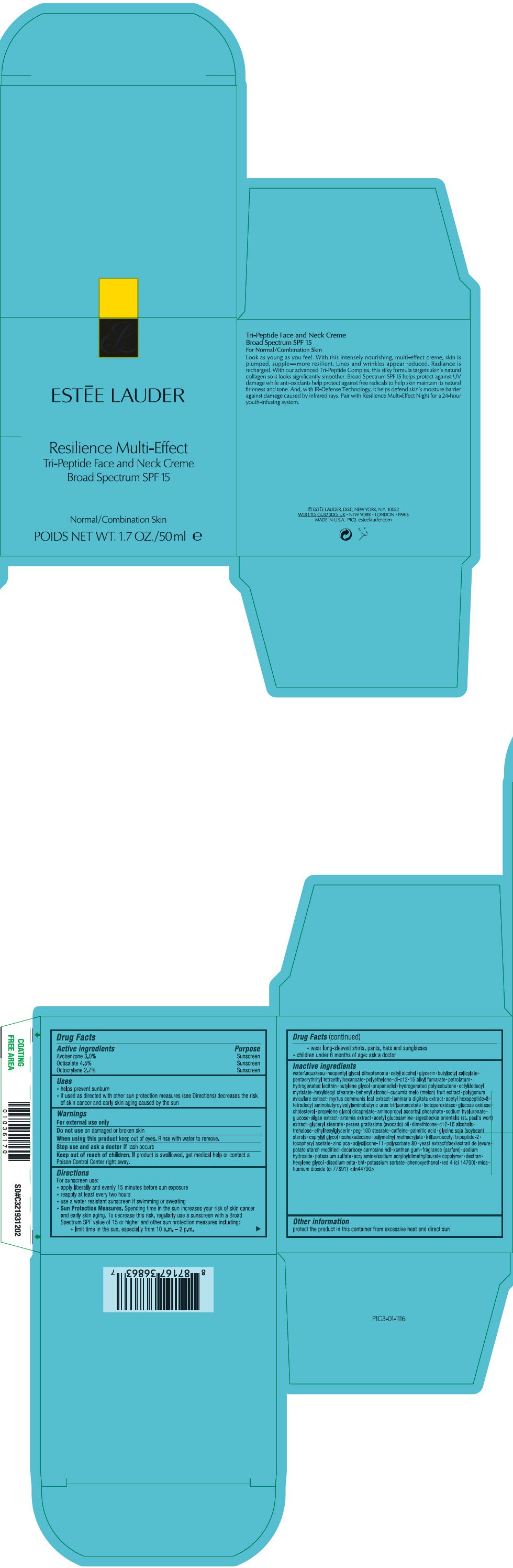
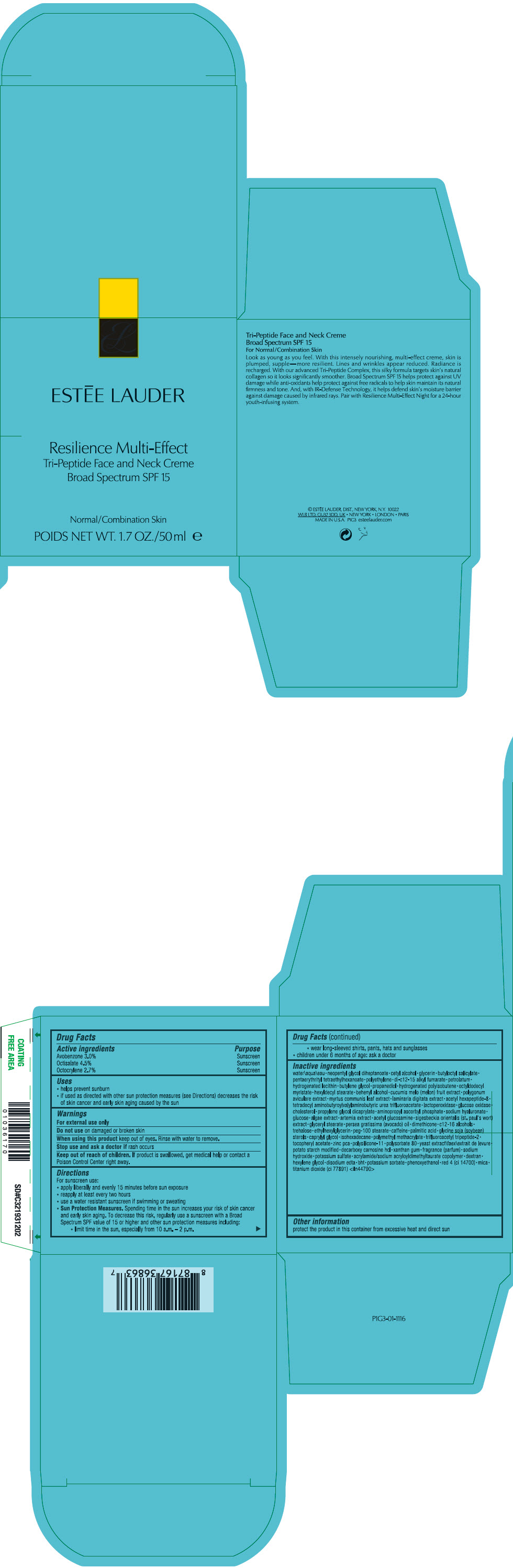
Label: RESILIENCE MULTI-EFFECT TRI-PEPTIDE FACE AND NECK CREME BROAD SPECTRUM SPF 15- avobenzone, octisalate, and octocrylene cream
-
NDC Code(s):
11559-052-01,
11559-052-02,
11559-052-03,
11559-052-04, view more11559-052-05, 11559-052-06
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • neopentyl glycol diheptanoate • cetyl alcohol • glycerin • butyloctyl salicylate • pentaerythrityl tetraethylhexanoate • polyethylene • di-c12-15 alkyl fumarate • petrolatum • hydrogenated lecithin • butylene glycol • propanediol • hydrogenated polyisobutene • octyldodecyl myristate • hexyldecyl stearate • behenyl alcohol • cucumis melo (melon) fruit extract • polygonum aviculare extract • myrtus communis leaf extract • laminaria digitata extract • acetyl hexapeptide-8 • tetradecyl aminobutyroylvalylaminobutyric urea trifluoroacetate • lactoperoxidase • glucose oxidase • cholesterol • propylene glycol dicaprylate • aminopropyl ascorbyl phosphate • sodium hyaluronate • glucose • algae extract • artemia extract • acetyl glucosamine • sigesbeckia orientalis (st. paul's wort) extract • glyceryl stearate • persea gratissima (avocado) oil • dimethicone • c12-16 alcohols • trehalose • ethylhexylglycerin • peg-100 stearate • caffeine • palmitic acid • glycine soja (soybean) sterols • caprylyl glycol • isohexadecane • polymethyl methacrylate • trifluoroacetyl tripeptide-2 • tocopheryl acetate • zinc pca • polysilicone-11 • polysorbate 80 • yeast extract\faex\extrait de levure • potato starch modified • decarboxy carnosine hcl • xanthan gum • fragrance (parfum) • sodium hydroxide • potassium sulfate • acrylamide/sodium acryloyldimethyltaurate copolymer • dextran • hexylene glycol • disodium edta • bht • potassium sorbate • phenoxyethanol • red 4 (ci 14700) • mica • titanium dioxide (ci 77891) <iln44790>
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Jar Carton
-
INGREDIENTS AND APPEARANCE
RESILIENCE MULTI-EFFECT TRI-PEPTIDE FACE AND NECK CREME BROAD SPECTRUM SPF 15
avobenzone, octisalate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-052 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERIN (UNII: PDC6A3C0OX) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PENTAERYTHRITYL TETRAETHYLHEXANOATE (UNII: XJ7052W897) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) DI-C12-15 ALKYL FUMARATE (UNII: A1CB3Z898P) PETROLATUM (UNII: 4T6H12BN9U) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPANEDIOL (UNII: 5965N8W85T) HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) OCTYLDODECYL MYRISTATE (UNII: S013N99GR8) HEXYLDECYL STEARATE (UNII: OJX2P28Y14) DOCOSANOL (UNII: 9G1OE216XY) MELON (UNII: 5CIT1X6U5V) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) MYELOPEROXIDASE (UNII: JQZ6YM58U5) GLUCOSE OXIDASE (UNII: 0T8392U5N1) CHOLESTEROL (UNII: 97C5T2UQ7J) PROPYLENE GLYCOL DICAPRYLATE (UNII: 581437HWX2) AMINOPROPYL ASCORBYL PHOSPHATE (UNII: 290O2PQ83R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) N-ACETYLGLUCOSAMINE (UNII: V956696549) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AVOCADO (UNII: SDS87L369F) DIMETHICONE (UNII: 92RU3N3Y1O) C12-16 ALCOHOLS (UNII: S4827SZE3L) TREHALOSE (UNII: B8WCK70T7I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PEG-100 STEARATE (UNII: YD01N1999R) CAFFEINE (UNII: 3G6A5W338E) PALMITIC ACID (UNII: 2V16EO95H1) SOY STEROL (UNII: PL360EPO9J) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ISOHEXADECANE (UNII: 918X1OUF1E) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ZINC PIDOLATE (UNII: C32PQ86DH4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STARCH, POTATO (UNII: 8I089SAH3T) DECARBOXY CARNOSINE HYDROCHLORIDE (UNII: 6X7K9I5QR7) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM HYDROXIDE (UNII: 55X04QC32I) POTASSIUM SULFATE (UNII: 1K573LC5TV) HEXYLENE GLYCOL (UNII: KEH0A3F75J) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) FD&C RED NO. 4 (UNII: X3W0AM1JLX) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-052-01 1 in 1 CARTON 11/01/2018 1 50 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:11559-052-02 1 in 1 CARTON 03/02/2022 2 75 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC:11559-052-03 1 in 1 CARTON 03/02/2022 3 30 mL in 1 JAR; Type 0: Not a Combination Product 4 NDC:11559-052-04 1 in 1 CARTON 03/02/2022 4 15 mL in 1 JAR; Type 0: Not a Combination Product 5 NDC:11559-052-05 7 mL in 1 PACKET; Type 0: Not a Combination Product 03/02/2022 11/01/2023 6 NDC:11559-052-06 7 mL in 1 JAR; Type 0: Not a Combination Product 11/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/01/2018 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(11559-052) , pack(11559-052) , label(11559-052) Establishment Name Address ID/FEI Business Operations NORTHTEC LLC 943871157 pack(11559-052) , label(11559-052) Establishment Name Address ID/FEI Business Operations NORTHTEC KEYSTONE 949264774 pack(11559-052) , label(11559-052) Establishment Name Address ID/FEI Business Operations PALC 078364654 pack(11559-052) , label(11559-052)