Label: LIDOCAINE HYDROCHLORIDE gel
-
NDC Code(s):
61010-5000-0,
61010-5000-1,
61010-5000-3,
61010-5000-4, view more61010-5000-5, 61010-5000-6
- Packager: Safetec of America, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL – 0.125 oz. packet
- PRINCIPAL DISPLAY PANEL – 4 oz. bottle
- Principal Display Panel - Pouch label
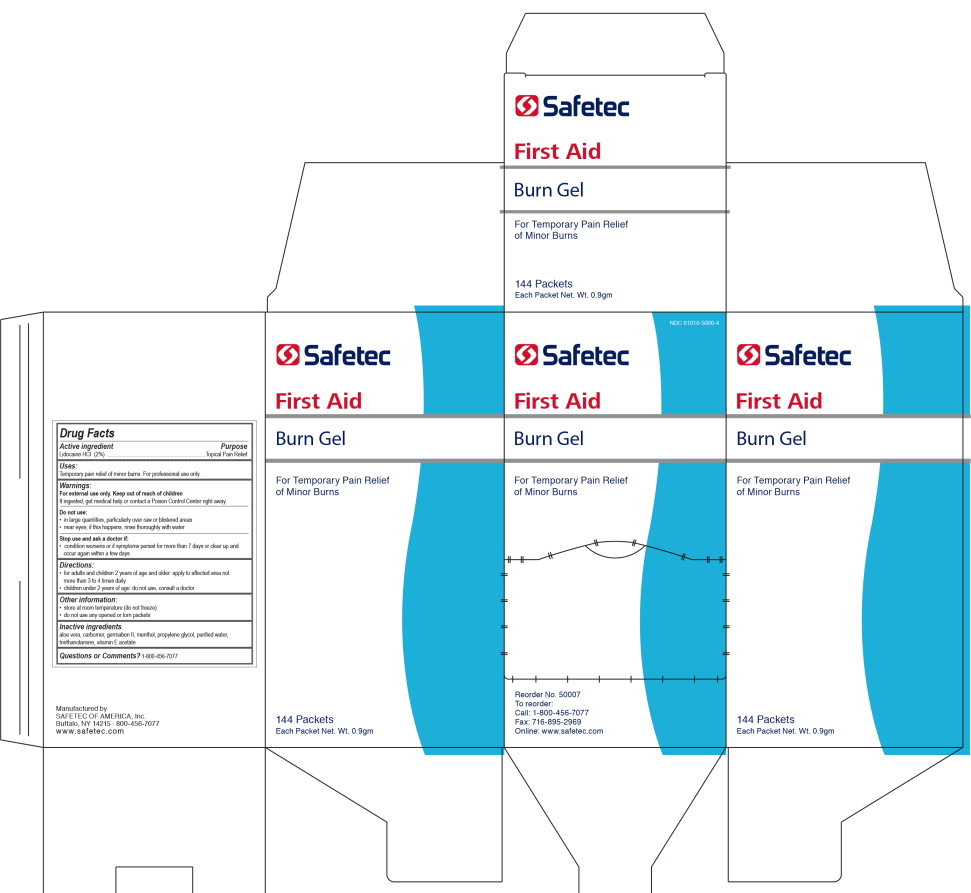
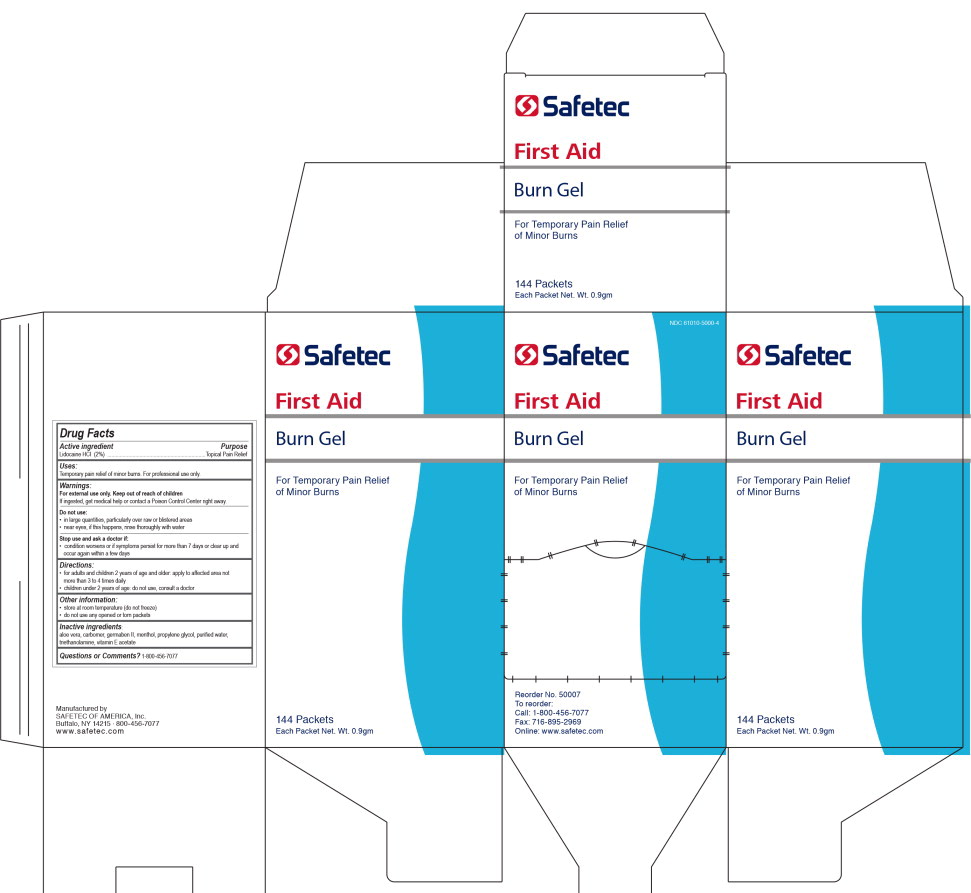
- Principal Display Panel - 144 Box label
- Principal Display Panel - 25 Box label
- Principal Display Panel – 10 Packet Box Label
-
INGREDIENTS AND APPEARANCE
LIDOCAINE HYDROCHLORIDE
lidocaine hydrochloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61010-5000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) WATER (UNII: 059QF0KO0R) MENTHOL (UNII: L7T10EIP3A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61010-5000-0 3.3 g in 1 PACKET; Type 0: Not a Combination Product 09/19/2012 2 NDC:61010-5000-1 121.2 g in 1 BOTTLE; Type 0: Not a Combination Product 09/19/2012 3 NDC:61010-5000-3 0.9 g in 1 POUCH; Type 0: Not a Combination Product 09/19/2012 4 NDC:61010-5000-4 144 in 1 CARTON 09/19/2012 4 0.9 g in 1 POUCH; Type 0: Not a Combination Product 5 NDC:61010-5000-5 25 in 1 CARTON 09/19/2012 5 0.9 g in 1 POUCH; Type 0: Not a Combination Product 6 NDC:61010-5000-6 10 in 1 BOX 04/01/2020 6 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 09/19/2012 Labeler - Safetec of America, Inc. (874965262) Establishment Name Address ID/FEI Business Operations Safetec of America, Inc. 874965262 manufacture(61010-5000)