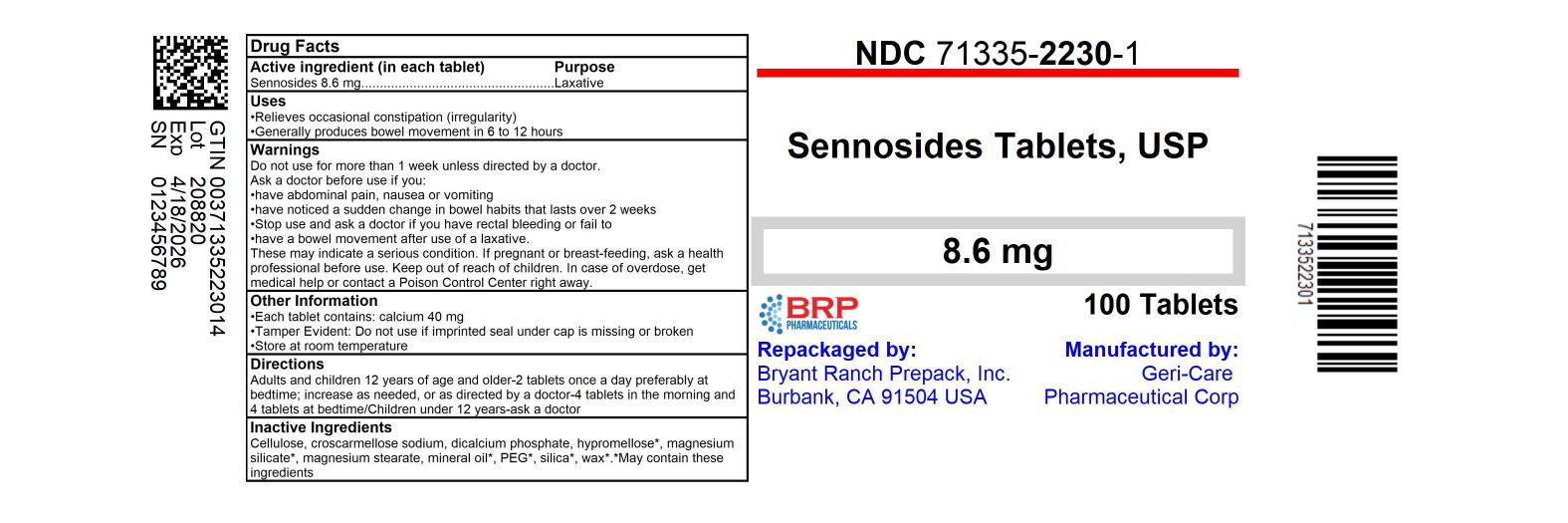
Label: GERI-KOT- sennosides tablet
-
NDC Code(s):
71335-2230-1,
71335-2230-2,
71335-2230-3,
71335-2230-4, view more71335-2230-5, 71335-2230-6, 71335-2230-7
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 57896-415
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
for more than 1 week unless directed by a doctor.
Ask a doctor before use if you
• have abdominal pain, nausea or vomiting
• have noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor ifyou have rectal bleeding
or fail to have a bowel movement after use of a laxative.
These may indicate a serious condition.
If pregnant or breast-feeding, ask a health professional before use. - Directions
- Other information
- Inactive ingredients
-
HOW SUPPLIED
Sennosides 8.6 mg
NDC: 71335-2230-1: 100 Tablets in a BOTTLE
NDC: 71335-2230-2: 60 Tablets in a BOTTLE
NDC: 71335-2230-3: 120 Tablets in a BOTTLE
NDC: 71335-2230-4: 56 Tablets in a BOTTLE
NDC: 71335-2230-5: 30 Tablets in a BOTTLE
NDC: 71335-2230-6: 90 Tablets in a BOTTLE
NDC: 71335-2230-7: 10 Tablets in a BOTTLE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GERI-KOT
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71335-2230(NDC:57896-415) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TALC (UNII: 7SEV7J4R1U) TRIACETIN (UNII: XHX3C3X673) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code PS23 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71335-2230-1 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2023 2 NDC:71335-2230-2 60 in 1 BOTTLE; Type 0: Not a Combination Product 09/07/2023 3 NDC:71335-2230-3 120 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2024 4 NDC:71335-2230-4 56 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2024 5 NDC:71335-2230-5 30 in 1 BOTTLE; Type 0: Not a Combination Product 09/18/2023 6 NDC:71335-2230-6 90 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2024 7 NDC:71335-2230-7 10 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 12/01/2022 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-2230) , RELABEL(71335-2230)