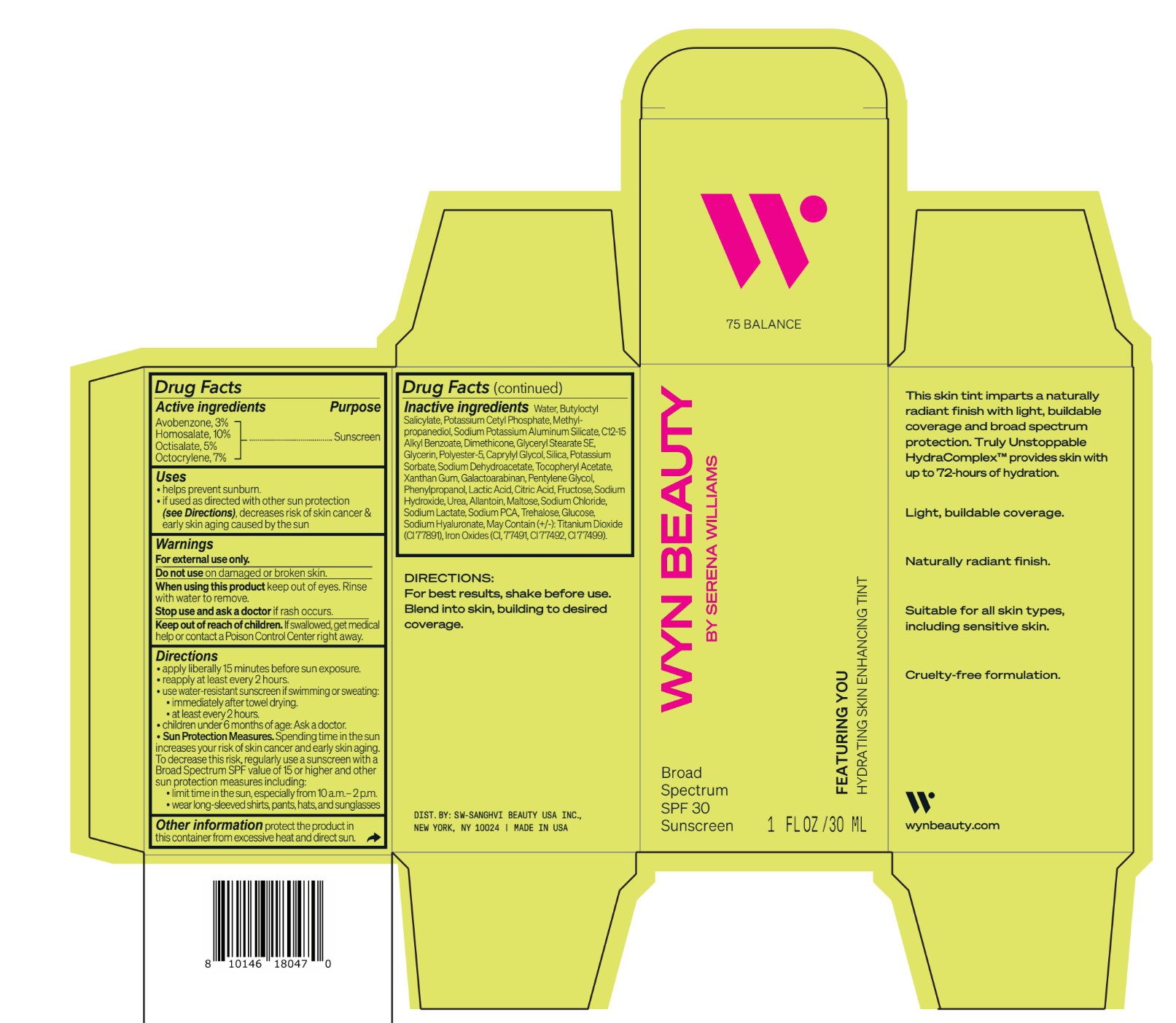
Label: CO-BB-54429 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-225 BECOME- avobenzone, homosalate,octisalate, octocrylene lotion
CO-BB-54367 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-315 CURIOSITY- avobenzone, homosalate,octisalate, octocrylene lotion
CO-BB-52816 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-390 ACHIEVE (avobenzone, homosalate,octisalate, octocry .......54438 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-45 STRIVE- avobenzone, homosalate,octisalate, octocrylene lotion
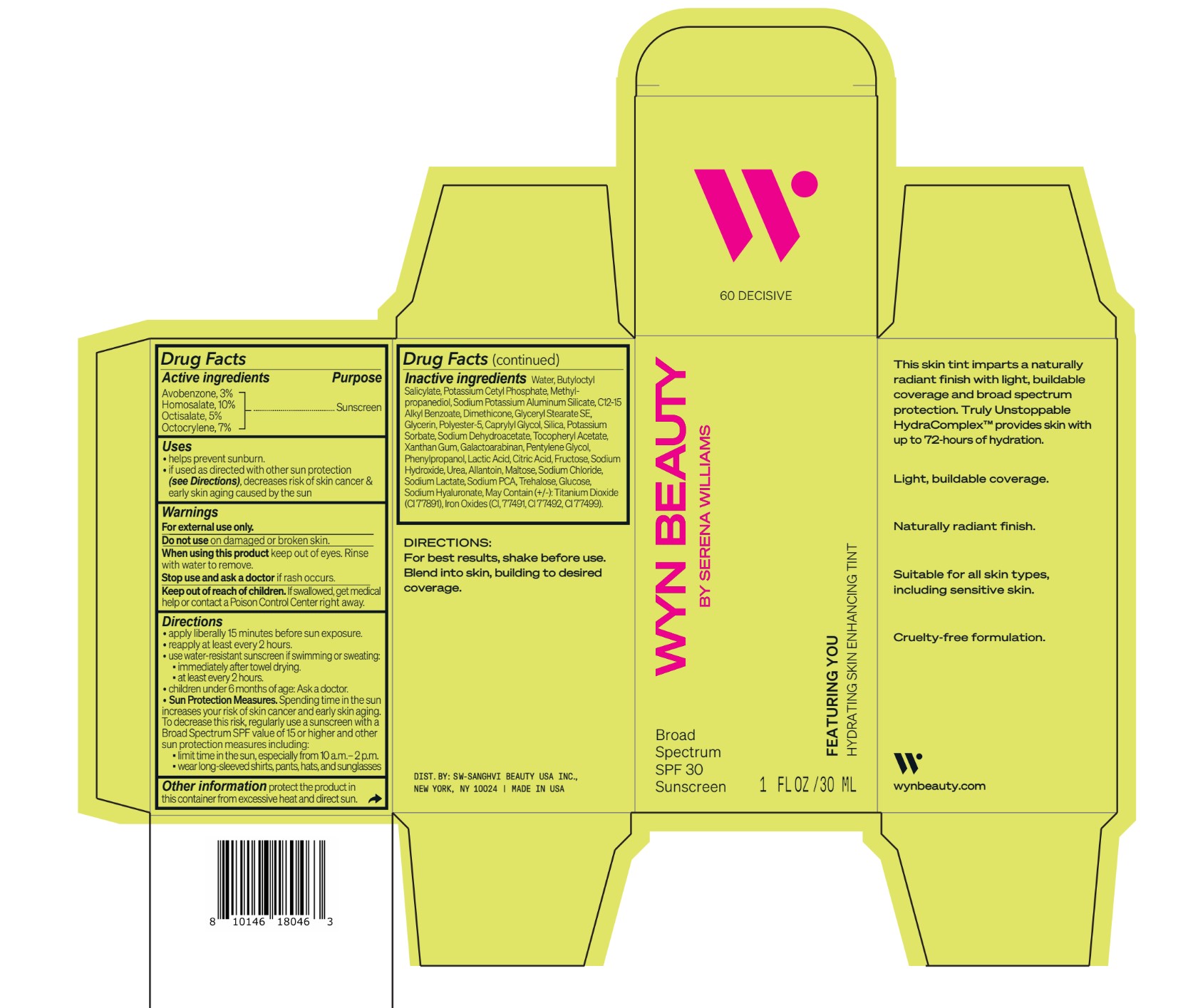
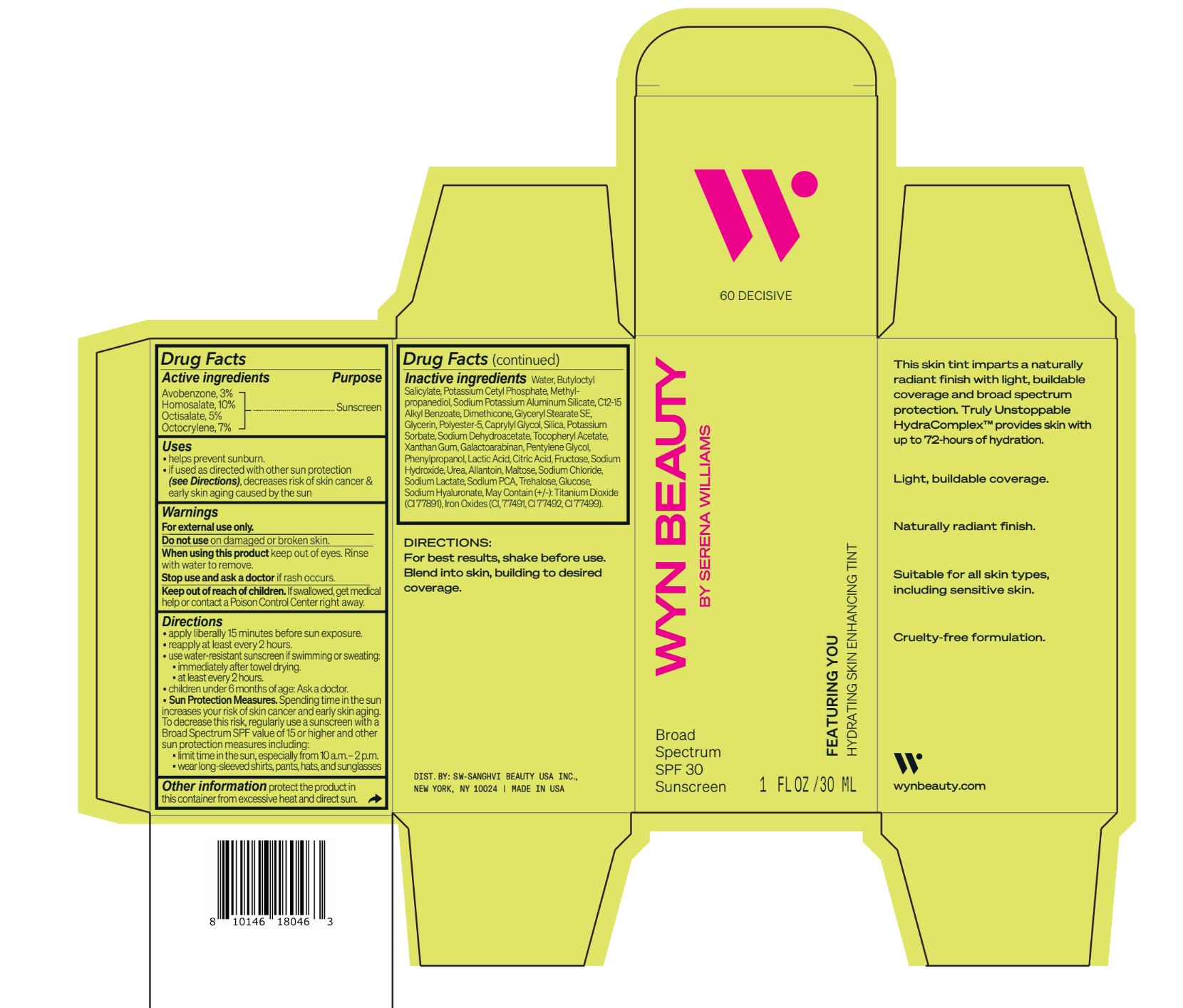
CO-BB-54436 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-60 DECISIVE- avobenzone, homosalate,octisalate, octocrylene lotion
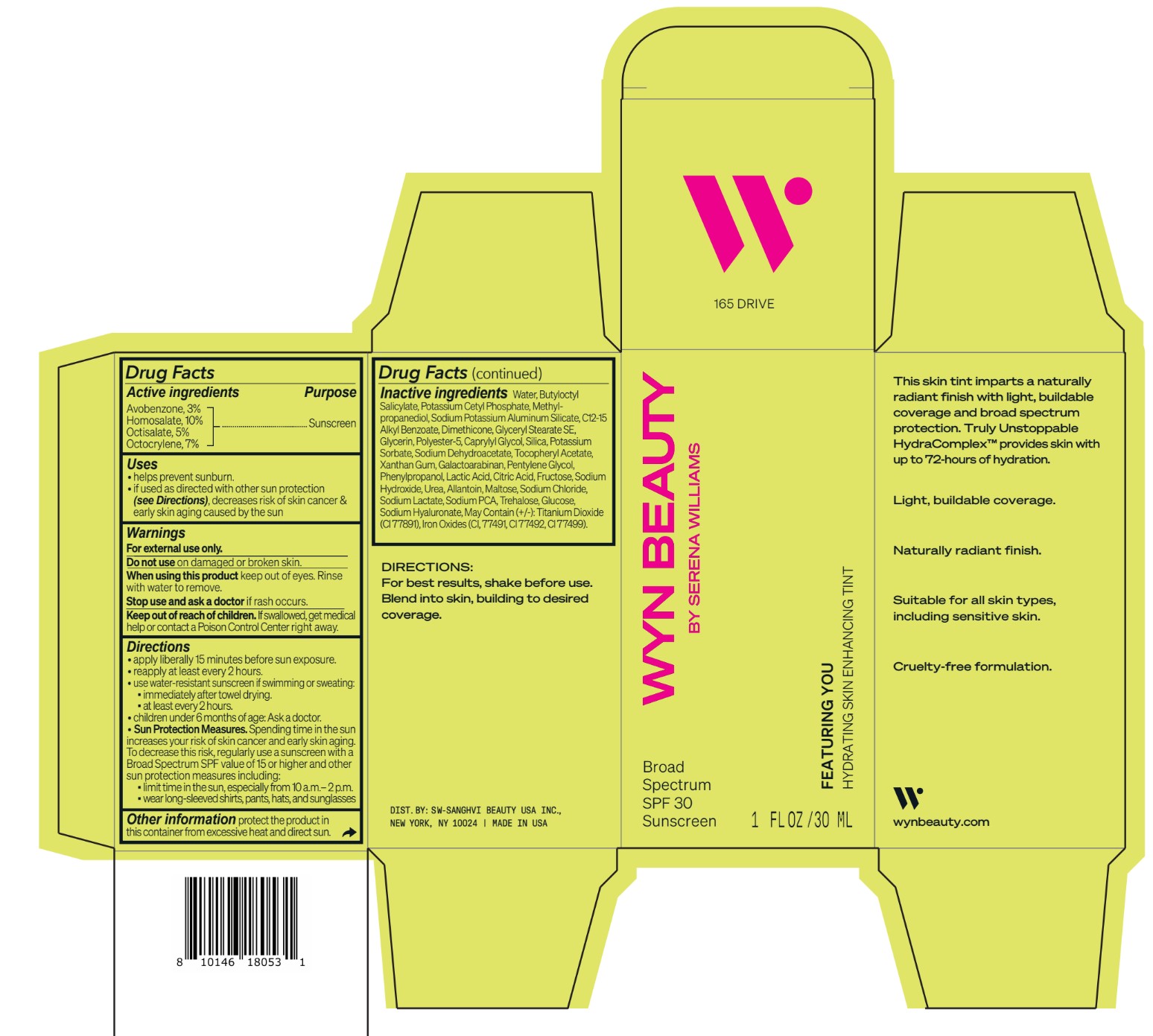
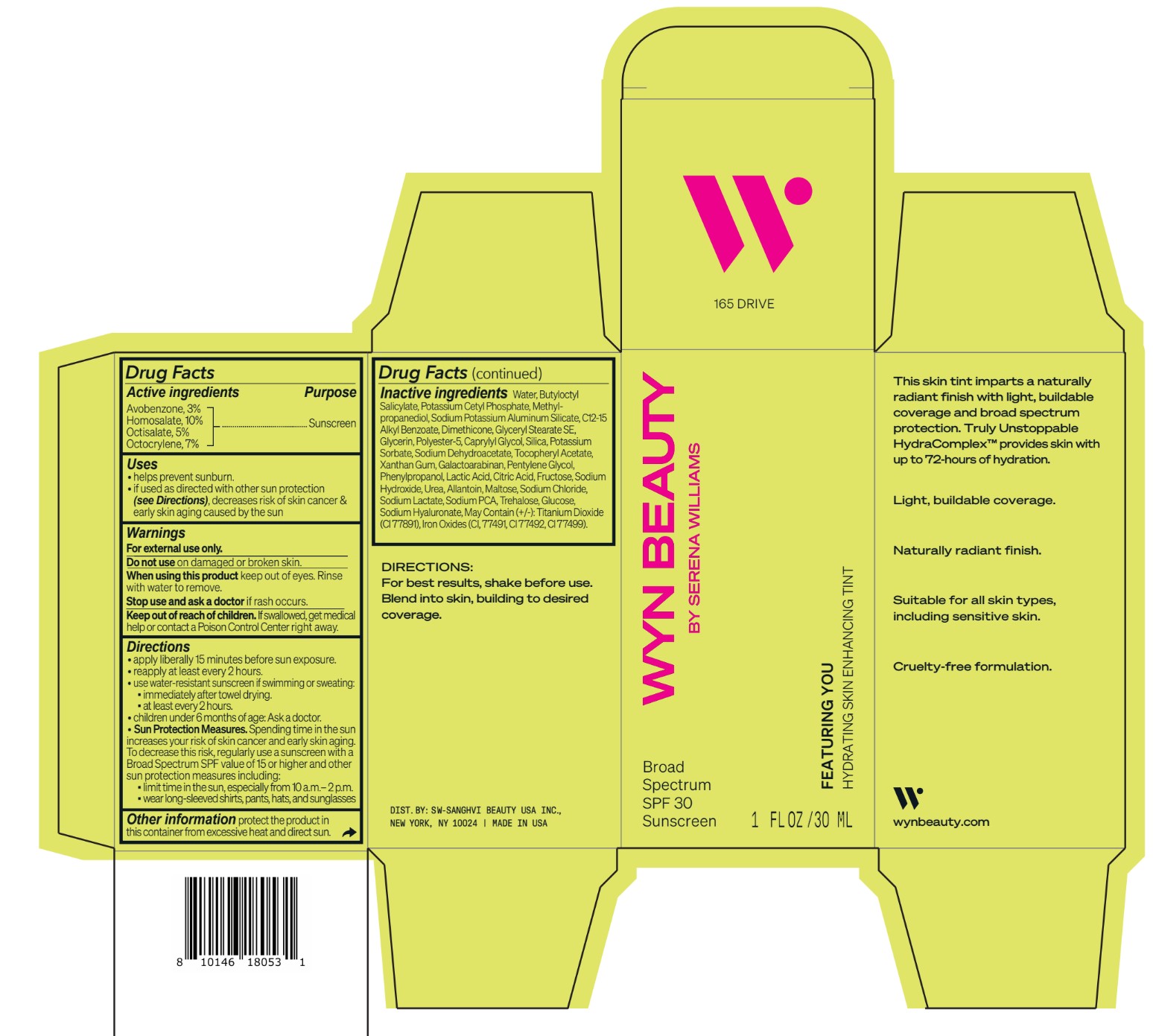
CO-BB-54441 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-165 DRIVE- avobenzone, homosalate,octisalate, octocrylene lotion
-
NDC Code(s):
84504-3001-1,
84504-3001-2,
84504-3002-1,
84504-3002-2, view more84504-3003-1, 84504-3003-2, 84504-3004-1, 84504-3004-2, 84504-3005-1, 84504-3005-2, 84504-3006-1, 84504-3006-2, 84504-3007-1, 84504-3007-2, 84504-3008-1, 84504-3008-2, 84504-3009-1, 84504-3009-2, 84504-3010-1, 84504-3010-2, 84504-3011-1, 84504-3011-2, 84504-3012-1, 84504-3012-2, 84504-3013-1, 84504-3013-2, 84504-3014-1, 84504-3014-2, 84504-3015-1, 84504-3015-2, 84504-3016-1, 84504-3016-2, 84504-3017-1, 84504-3017-2, 84504-3018-1, 84504-3018-2, 84504-3019-1, 84504-3019-2, 84504-3020-1, 84504-3020-2, 84504-3021-1, 84504-3021-2, 84504-3022-1, 84504-3022-2, 84504-3023-1, 84504-3023-2, 84504-3024-1, 84504-3024-2, 84504-3025-1, 84504-3025-2, 84504-3026-1, 84504-3026-2, 84504-3027-1, 84504-3027-2, 84504-3028-1, 84504-3028-2, 84504-3029-1, 84504-3029-2, 84504-3030-1, 84504-3030-2, 84504-3031-1, 84504-3031-2, 84504-3032-1, 84504-3032-2, 84504-3033-1, 84504-3033-2, 84504-3034-1, 84504-3034-2, 84504-3035-1, 84504-3035-2, 84504-3036-1, 84504-3036-2
- Packager: SW-SANGHVI BEAUTY USA, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DOSAGE & ADMINISTRATION
Directions
• apply liberally 15 minutes before sun exposure.
•reapply at least every 2 hours.
• use water-resistant sunscreen if swimming or sweating:
▪immediately after towel drying.
▪ at least every 2 hours.
• children under 6 months of age: Ask a doctor.
• Sun Protection Measures. Spending time in the sun
increases your risk of skin cancer and early skin aging.
To decrease this risk, regularly use a sunscreen with a
Broad Spectrum SPF value of 15 or higher and other
sun protection measures including:
▪limit time in the sun, especially from 10 a.m.– 2 p.m.
▪ wear long-sleeved shirts, pants, hats, and sunglasses
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
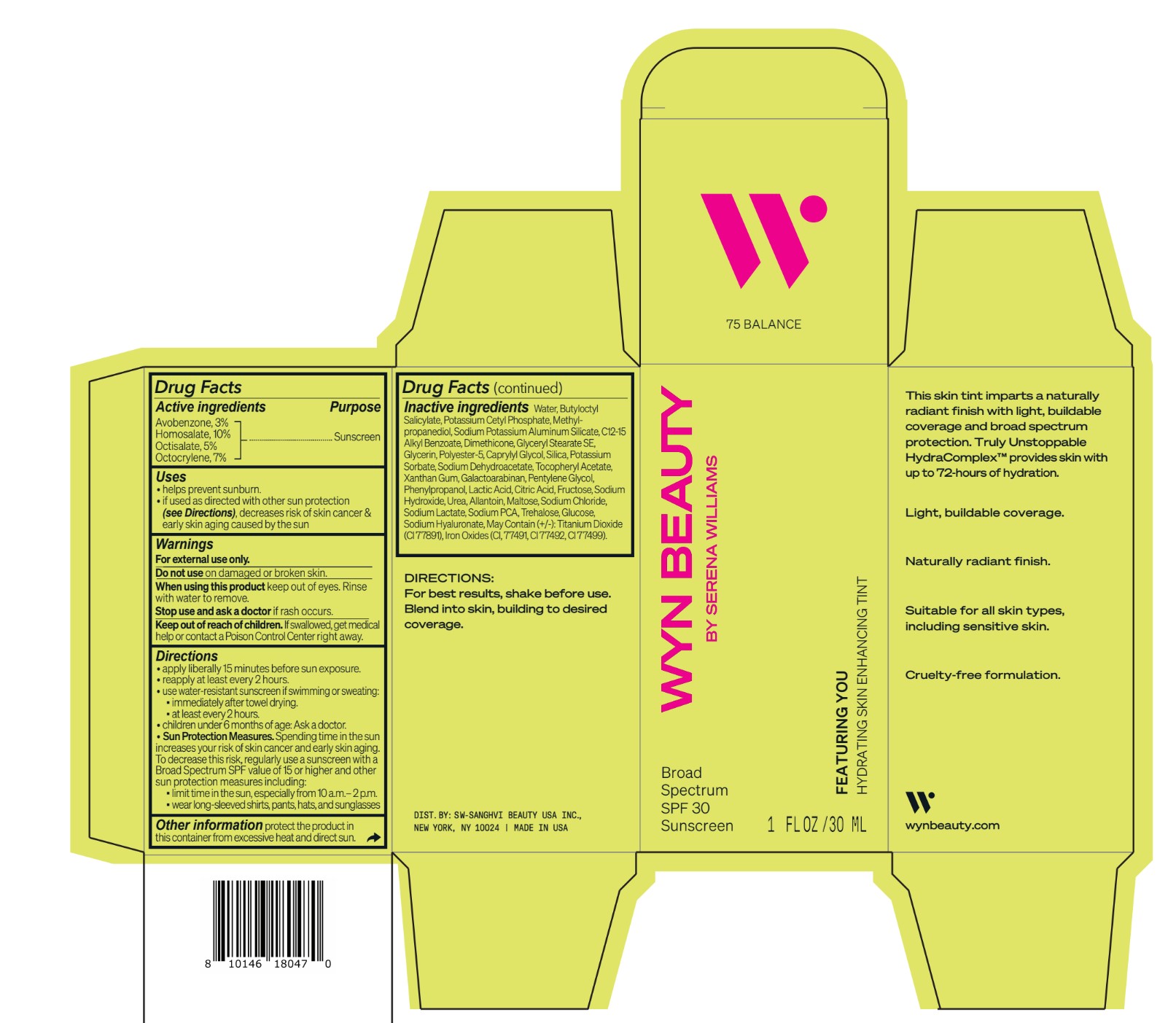
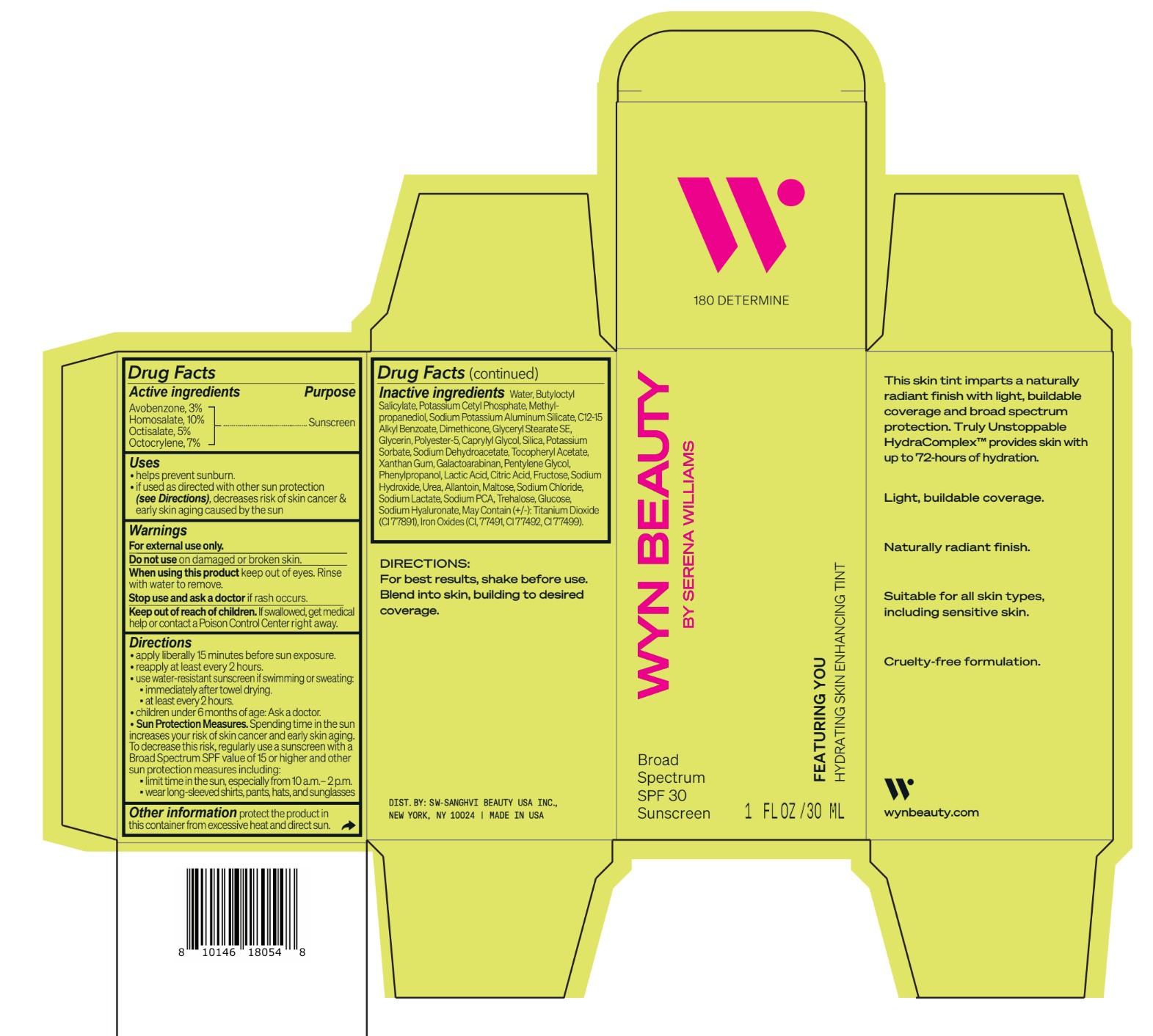
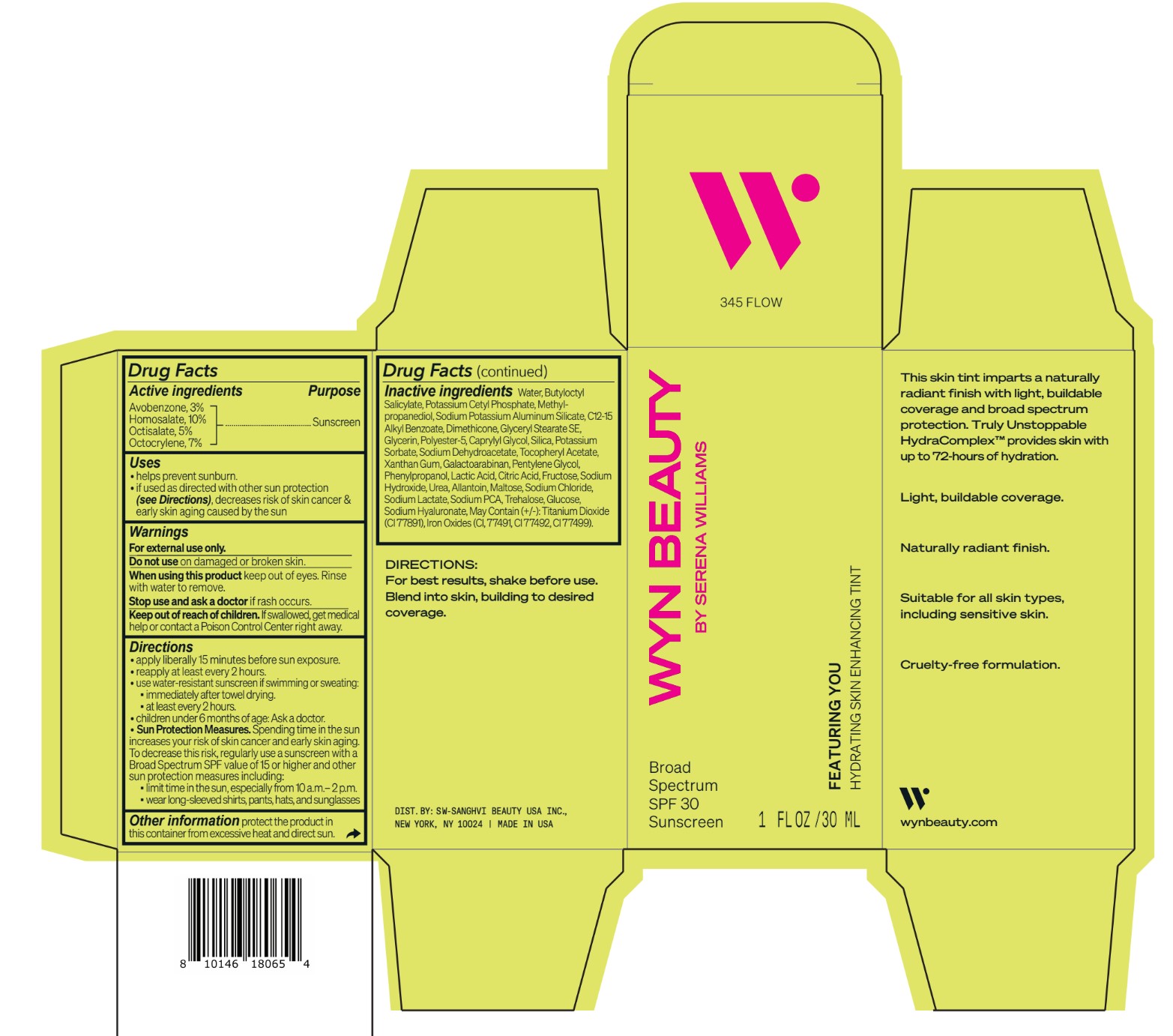
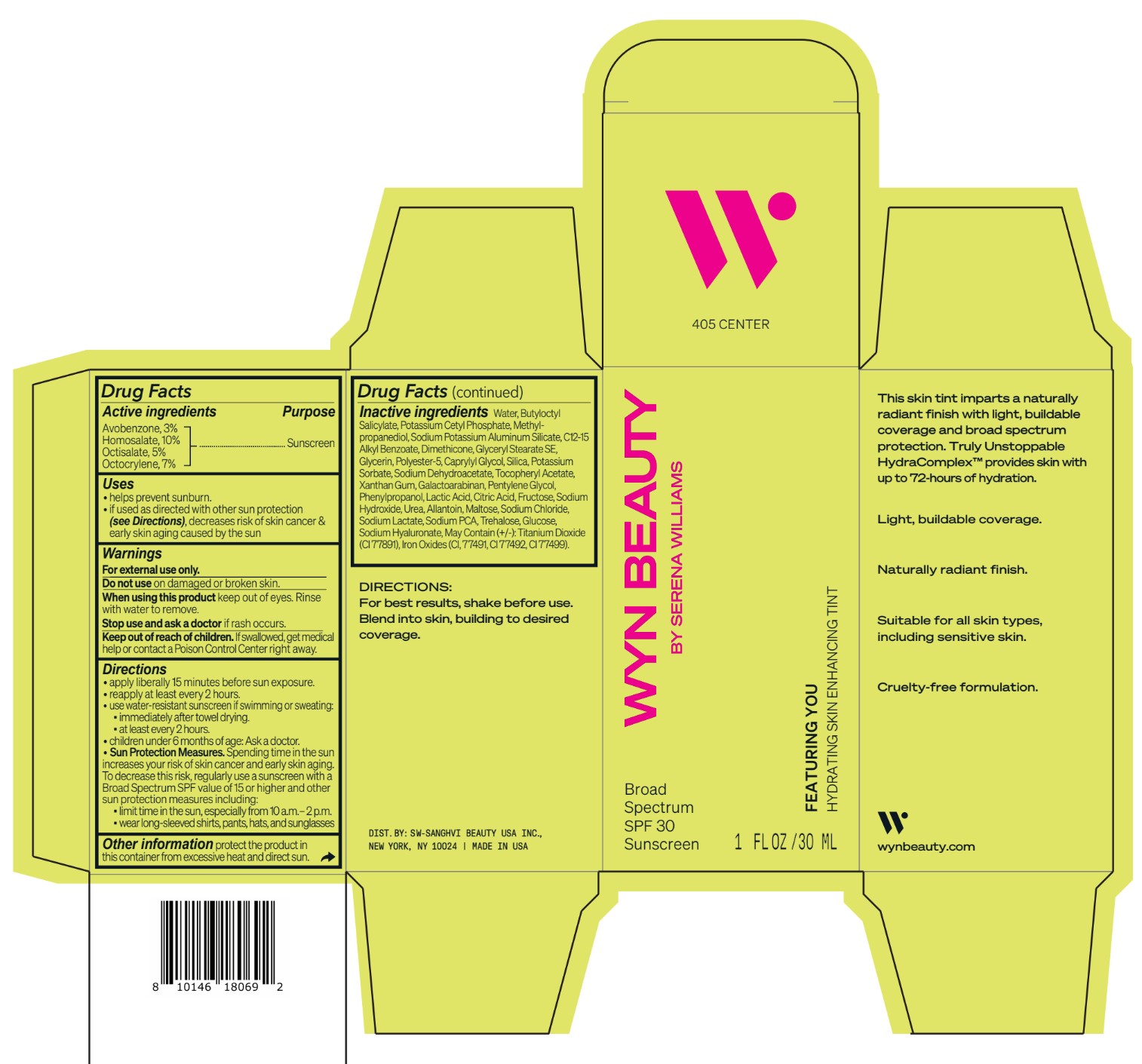
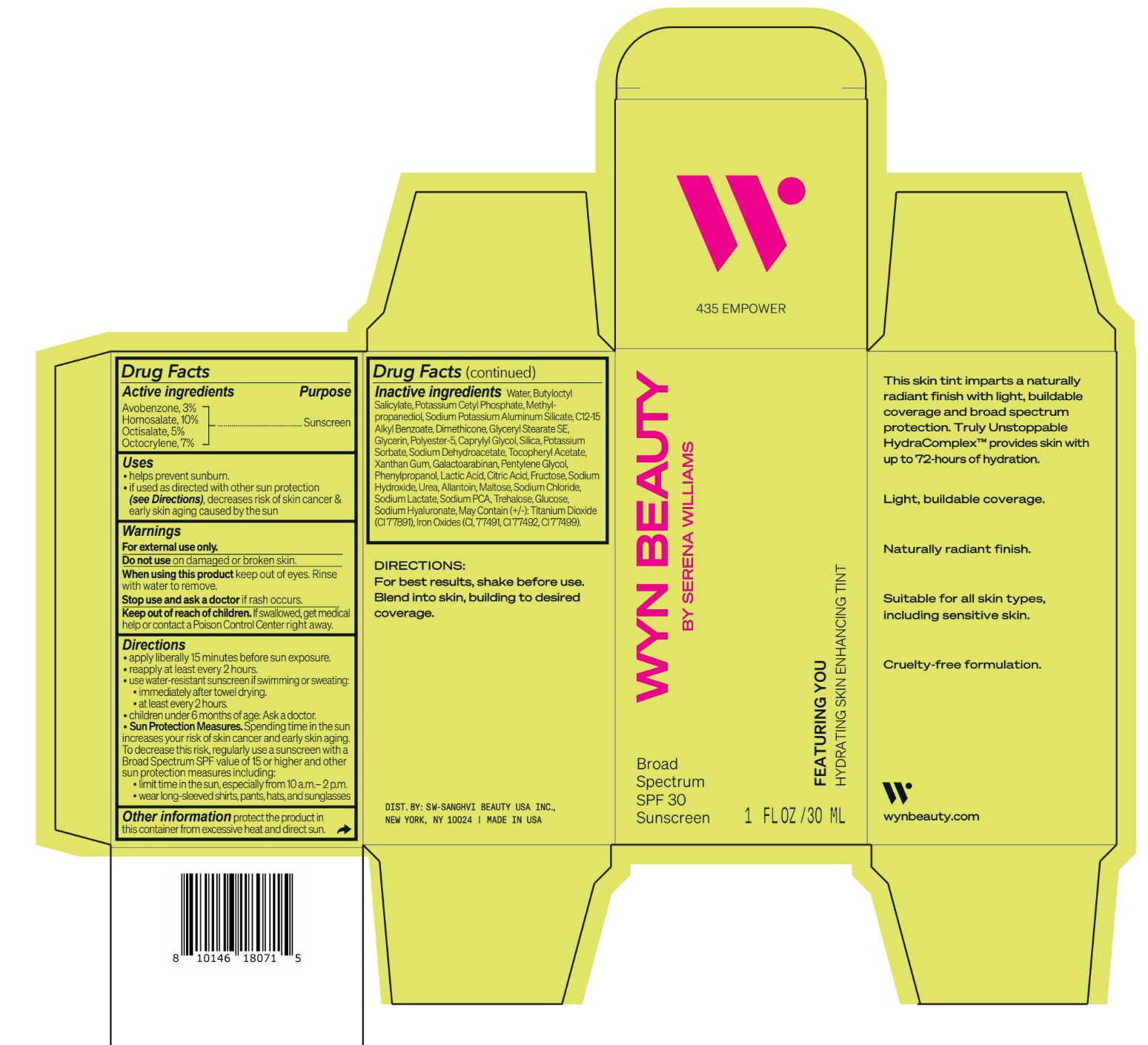
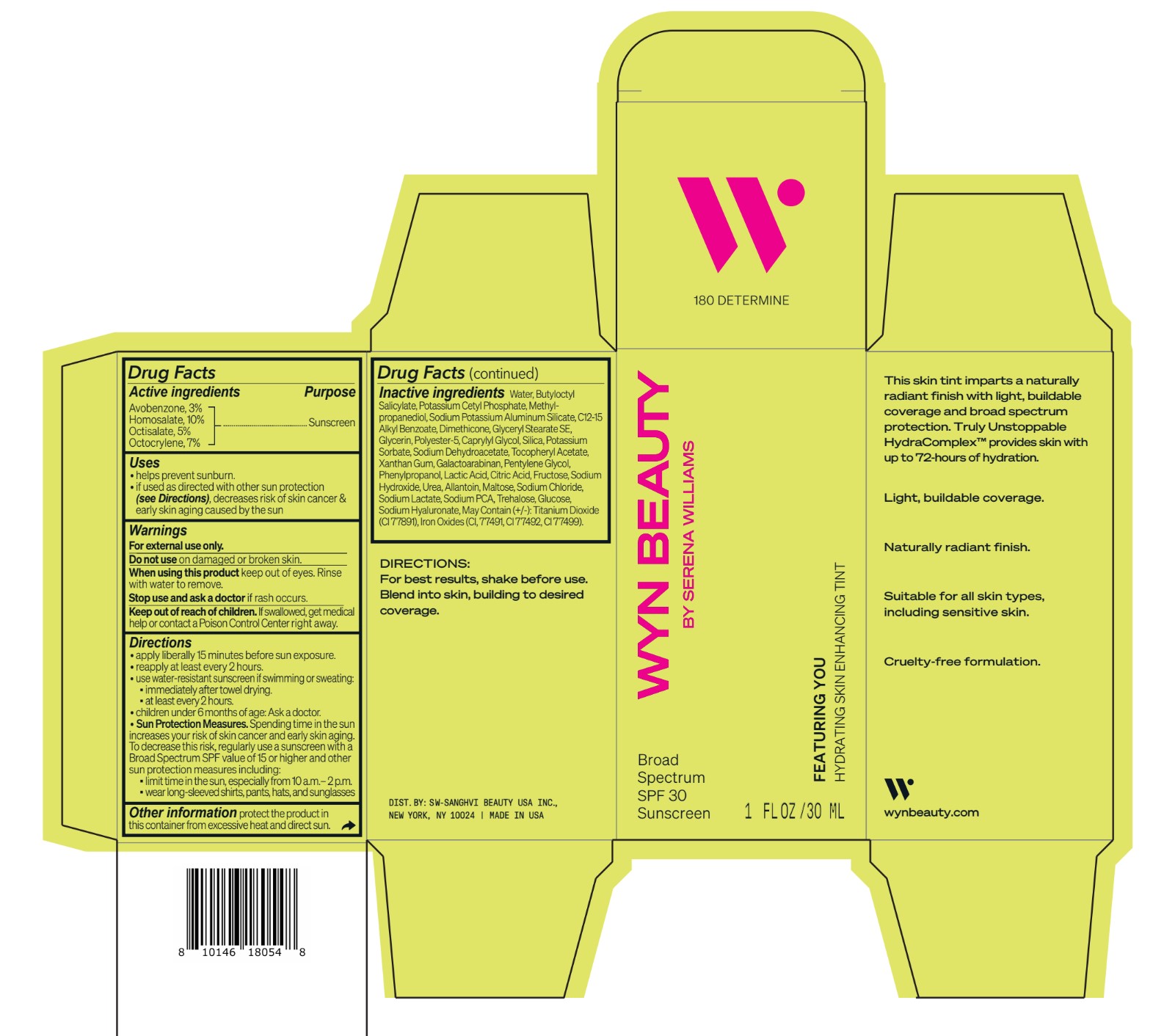
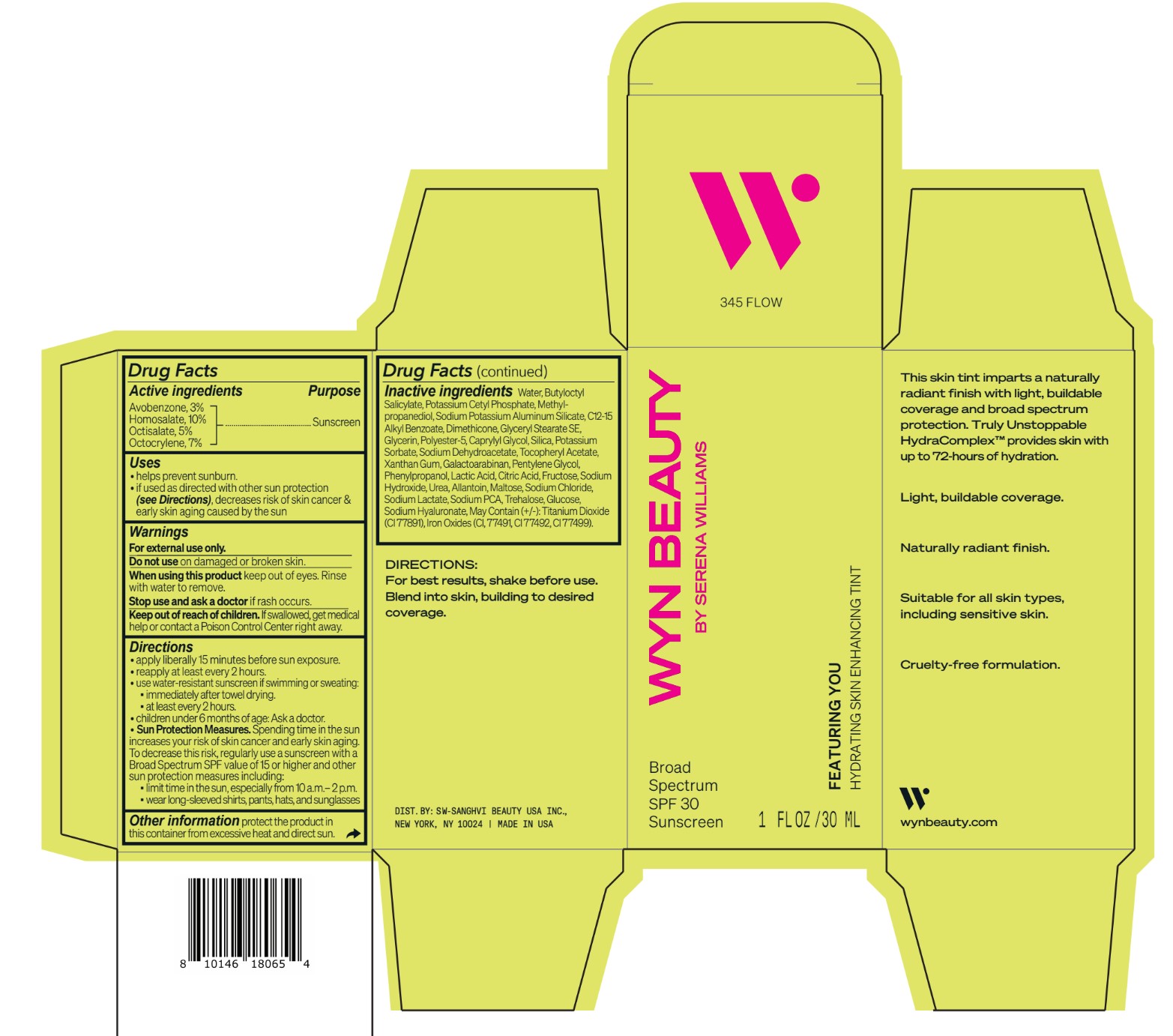
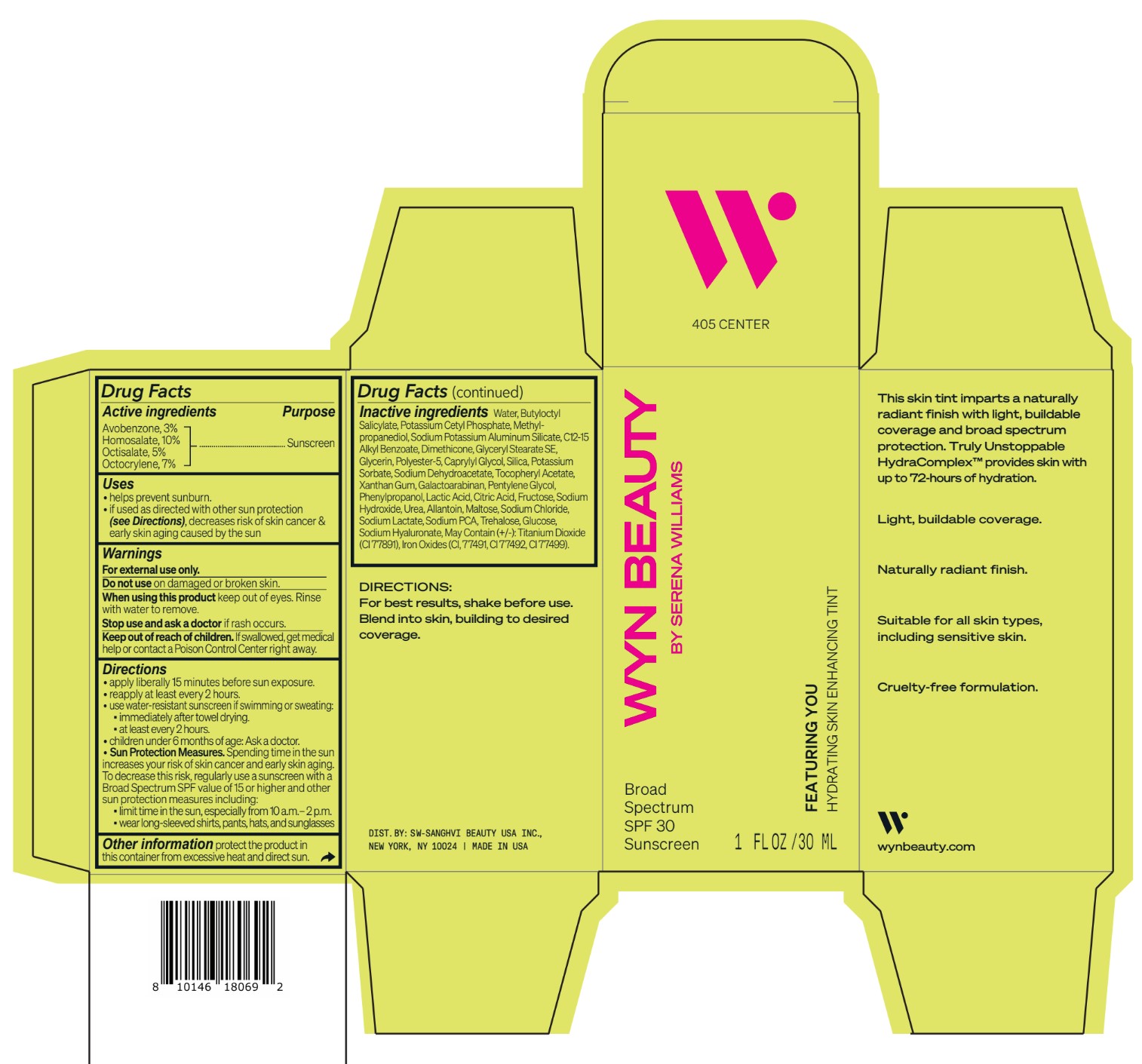
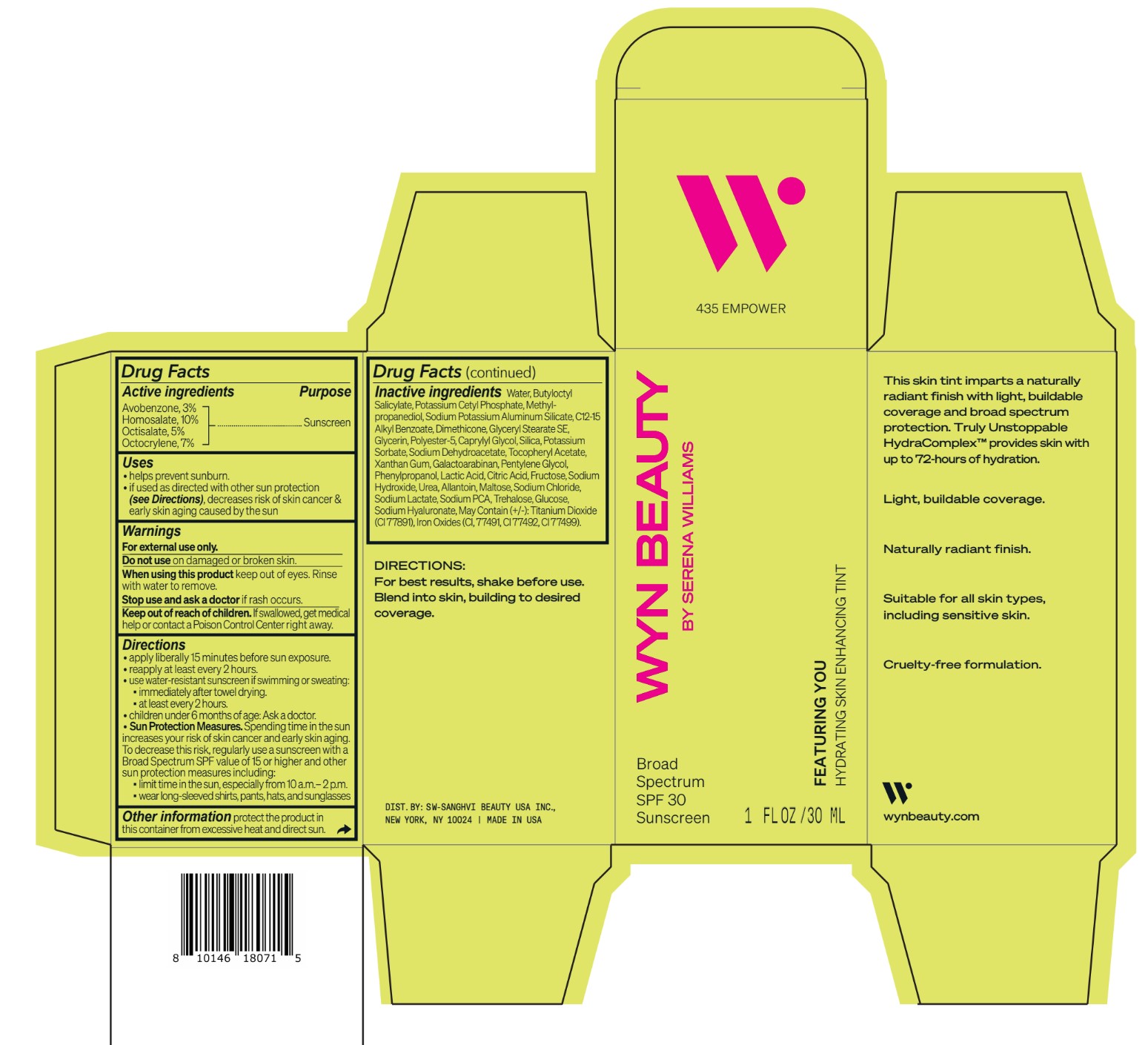
Inactive ingredients Water, Butyloctyl Salicylate, Potassium Cetyl Phosphate, Methylpropanediol, Sodium Potassium Aluminum Silicate, C12-15 Alkyl Benzoate, Dimethicone, Glyceryl Stearate SE, Glycerin, Polyester-5, Caprylyl Glycol, Silica, Potassium Sorbate, Sodium Dehydroacetate, Tocopheryl Acetate, Xanthan Gum, Galactoarabinan, Pentylene Glycol, Phenylpropanol, Lactic Acid, Citric Acid, Fructose, Sodium Hydroxide, Urea, Allantoin, Maltose, Sodium Chloride, Sodium Lactate, Sodium PCA, Trehalose, Glucose, Sodium Hyaluronate, May Contain (+/-): Titanium Dioxide (CI 77891), Iron Oxides (CI, 77491, CI 77492, CI 77499).

- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- WARNINGS
- Featuring You Hydrating Skin Enhancing Tint SPF30
-
INGREDIENTS AND APPEARANCE
CO-BB-54429 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-225 BECOME
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3015-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3015-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54367 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-315 CURIOSITY
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3021-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3021-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-52816 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-390 ACHIEVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3026 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3026-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3026-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54342 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-495 DISCOVER
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3033-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3033-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54420 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-195 STEP
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3013-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3013-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54382 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-210 COMMIT
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3014-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3014-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-55451 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-540 ENERGY
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3036-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3036-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54432 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-30 INSIST
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3002-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3002-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54426 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-240 VELOCITY
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3016-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3016-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54383 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-405 CENTER
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3027-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3027-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54366 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-330 PINNACLE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3022-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3022-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54341 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-525 SHINE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3035-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3035-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54431 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-270 FURTHER
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3018-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3018-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54368 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-360 NEXT
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3024-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3024-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54378 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-480 BUILD
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3032-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3032-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54442 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-135 MORE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3009-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3009-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54339 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-435 EMPOWER
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3029-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3029-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-55450 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-15 EXPLORE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) WATER (UNII: 059QF0KO0R) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3001-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3001-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54443 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-105 BEING
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3007-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3007-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54439 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-150 FLEX
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3010-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3010-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54417 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-180 DETERMINE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3012-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3012-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-53653 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-300 AWARE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3020-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3020-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-52549 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-375 STRENGTH
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3025-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3025-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54386 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-450 ABOVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3030-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3030-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-53750 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-120 REALIZE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3008-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3008-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54416 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-255 OPEN
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3017-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3017-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54413 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-285 POSSIBLE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3019-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3019-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54369 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-345 FLOW
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3023-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3023-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54345 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-420 RELEASE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3028-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3028-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54340 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-465 MOVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3031-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3031-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54343 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-510 MOMENTUM
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3034-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3034-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54433 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-90 REACH
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3006-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3006-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54435 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-75 BALANCE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3005-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3005-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54438 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-45 STRIVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3003-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3003-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54436 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-60 DECISIVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3004-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3004-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 CO-BB-54441 FEATURING YOU HYDRATING SKIN ENHANCING TINT SPF30-165 DRIVE
avobenzone, homosalate,octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84504-3011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength PHENYLPROPANOL (UNII: 0F897O3O4M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) POLYESTER-5 (TG-38) (UNII: 2L9351NW8W) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PENTYLENE GLYCOL (UNII: 50C1307PZG) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ALLANTOIN (UNII: 344S277G0Z) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FRUCTOSE (UNII: 6YSS42VSEV) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) SODIUM POTASSIUM ALUMINUM SILICATE (UNII: Z6C6FF3Y9R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) XANTHAN GUM (UNII: TTV12P4NEE) GALACTOARABINAN (UNII: SL4SX1O487) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM LACTATE (UNII: TU7HW0W0QT) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SODIUM HYDROXIDE (UNII: 55X04QC32I) UREA (UNII: 8W8T17847W) MALTOSE ANHYDROUS (UNII: 66Y63L379N) TREHALOSE (UNII: B8WCK70T7I) GLYCERIN (UNII: PDC6A3C0OX) METHYLPROPANEDIOL (UNII: N8F53B3R4R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84504-3011-2 1 in 1 CARTON 04/01/2024 1 NDC:84504-3011-1 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 04/01/2024 Labeler - SW-SANGHVI BEAUTY USA, INC. (118985236) Registrant - HB REGULATORY SOLUTIONS LLC (117575206)