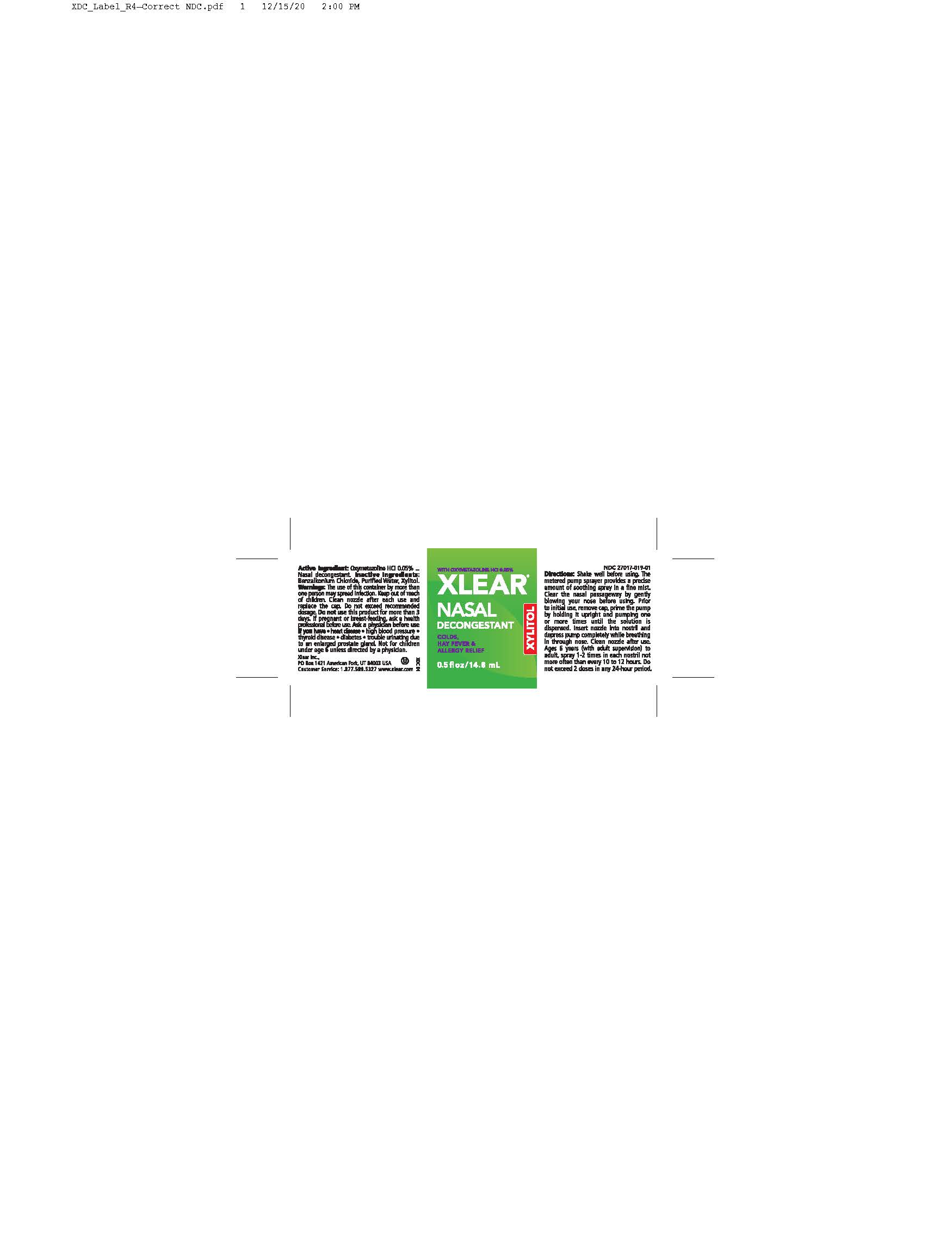
Label: XLEAR NASAL DECONGESTANT- nasal spray liquid
- NDC Code(s): 27017-018-01
- Packager: Xlear Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNINGS AND PRECAUTIONS
The use of this container by morethan one person may spread infection. Keep out of reach of children. Clean nozzle after each use and replace the cap. Do not exceed recommended dosage. Do not use this product for more than 3 days. If pregnant or breast-feeding, ask a health professional before use. Ask a physician before use if you have, heart disease, highblood pressure, thyroid disease, diabetes, trouble unitnating due to enlarged prostate gland. Not for children under age 6 unless directed by a physician.

- PURPOSE
-
INDICATIONS & USAGE
Shake well before using. The metered pump sprayer provides a precise amount of soothing spray in a fine mist. Claer the nasal passage-way by gently blowing your nose before using. Prior to initial use, remove cap, prime the pump by holding it upright and pumping one or more times until the solution is dispensed. Insert nozzle into nostirl and depress pump completely while breathing in through the nose. Clean nozzle after use. Ages 6 years to adult (with adult supervision) - spray 1-2 times in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS
The use of this container by more than one person may spread infection. Keep out of reach of children. Clean nozzle after each use and replace the cap. Do not exceed recommended dosage. Do not use this product for more than 3 days. If pregnant or breast-feeding, ask a health professional before use. Ask a physician before use if you have, heart disease, highblood pressure, thyroid disease, diabetes, trouble unitnating due to enlarged prostate gland. Not for children under age 6 unless directed by a physician.
- ACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Shake well before using. The metered pump sprayer provides a precise amount of soothing spray in a fine mist. Claer the nasal passage-way by gently blowing your nose before using. Prior to initial use, remove cap, prime the pump by holding it upright and pumping one or more times until the solution is dispensed. Insert nozzle into nostirl and depress pump completely while breathing in through the nose. Clean nozzle after use. Ages 6 years to adult (with adult supervision) - spray 1-2 times in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- INACTIVE INGREDIENT
-
PRINCIPAL DISPLAY PANEL
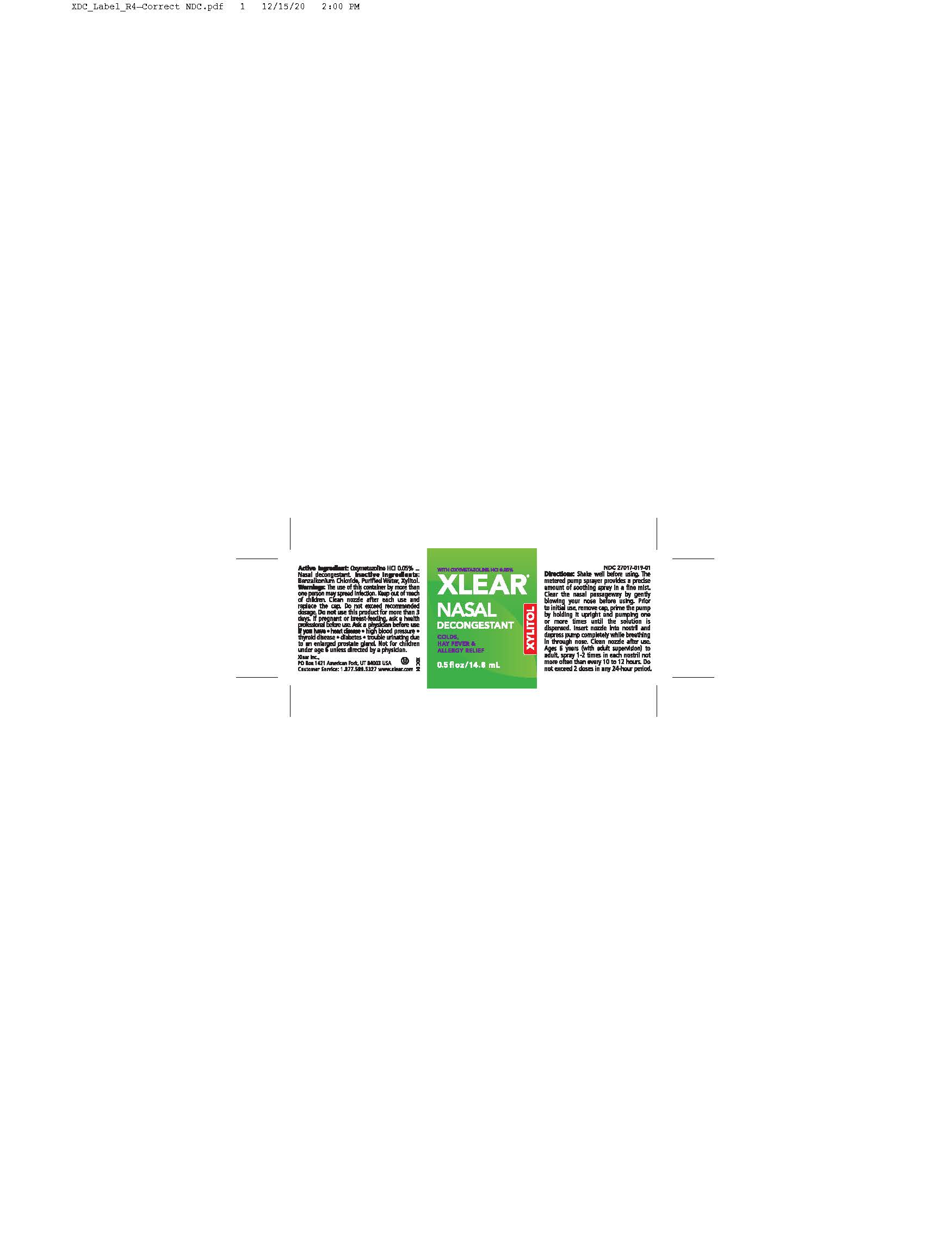

The use of this container by more than one person may spread infection. Keep out of reach of children. Clean nozzle after each use and replace the cap. Do not exceed recommended dosage. Do not use this product for more than 3 days. If pregnant or breast-feeding, ask a health professional before use. Ask a physician before use if you have, heart disease, highblood pressure, thyroid disease, diabetes, trouble unitnating due to enlarged prostate gland. Not for children under age 6 unless directed by a physician.
-
INGREDIENTS AND APPEARANCE
XLEAR NASAL DECONGESTANT
nasal spray liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:27017-018 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.0005 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) XYLITOL (UNII: VCQ006KQ1E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27017-018-01 1 g in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 03/12/2019 Labeler - Xlear Inc. (839884058) Registrant - Xlear Inc. (839884058) Establishment Name Address ID/FEI Business Operations Xlear Inc. 839884058 manufacture(27017-018)