Label: FUNGIFREE NAIL REPAIR SOLUTION- tolnaftate ointment
- NDC Code(s): 83364-008-01
- Packager: YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
- This is a repackaged label.
- Source NDC Code(s): 84010-013
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
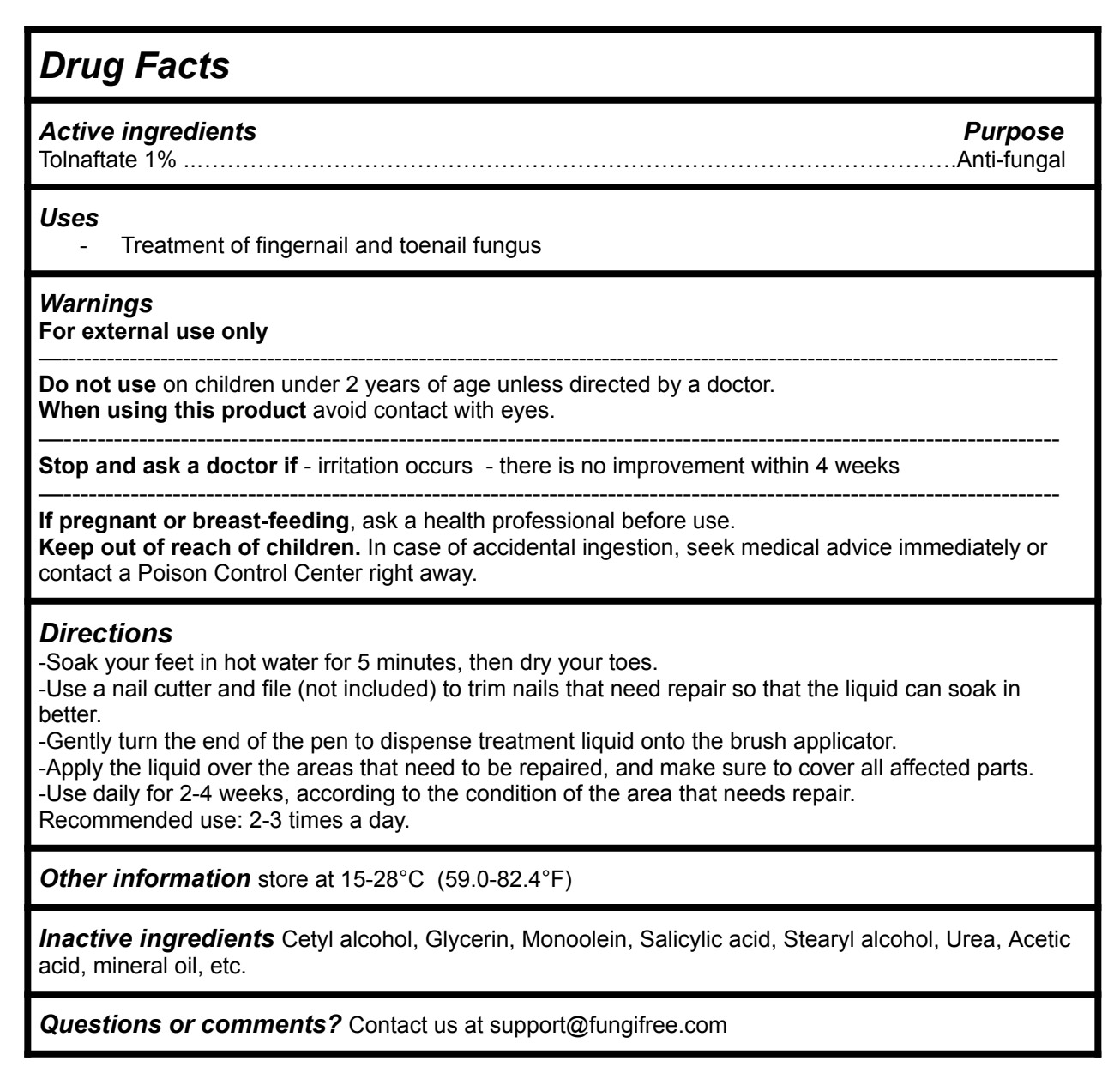
Drug Facts
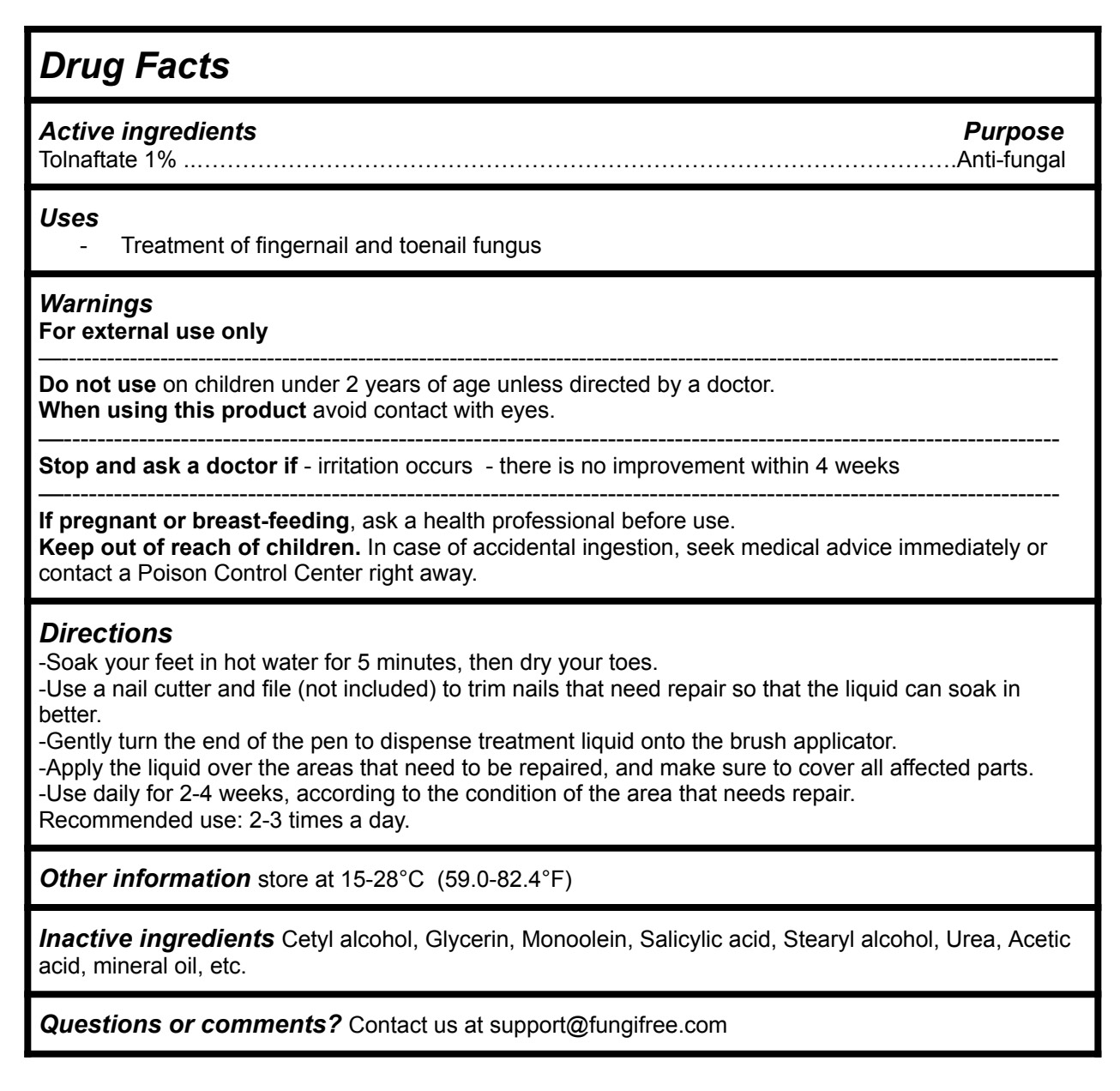
Directions
-Soak your feet in hot water for 5 minutes, then dry your toes.
-Use a nail cutter and file (not included) to trim nails that need repair so that the liquid can soak in better.
-Gently turn the end of the pen to dispense treatment liquid onto the brush applicator.
-Apply the liquid over the areas that need to be repaired, and make sure to cover all affected parts.
-Use daily for 2-4 weeks, according to the condition of the area that needs repair.
Recommended use: 2-3 times a day.
-
INGREDIENTS AND APPEARANCE
FUNGIFREE NAIL REPAIR SOLUTION
tolnaftate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83364-008(NDC:84010-013) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) UREA (UNII: 8W8T17847W) ACETIC ACID (UNII: Q40Q9N063P) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) SALICYLIC ACID (UNII: O414PZ4LPZ) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83364-008-01 1 in 1 BOX 06/25/2024 1 30 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/25/2024 Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463)