Label: 16BKOUTY NALL FUNGUS RENEWAL liquid
- NDC Code(s): 84422-101-01
- Packager: Shenzhen Graceful Cosmetics Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS
- CLEANING, DISINFECTING, AND STERILIZATION INSTRUCTIONS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
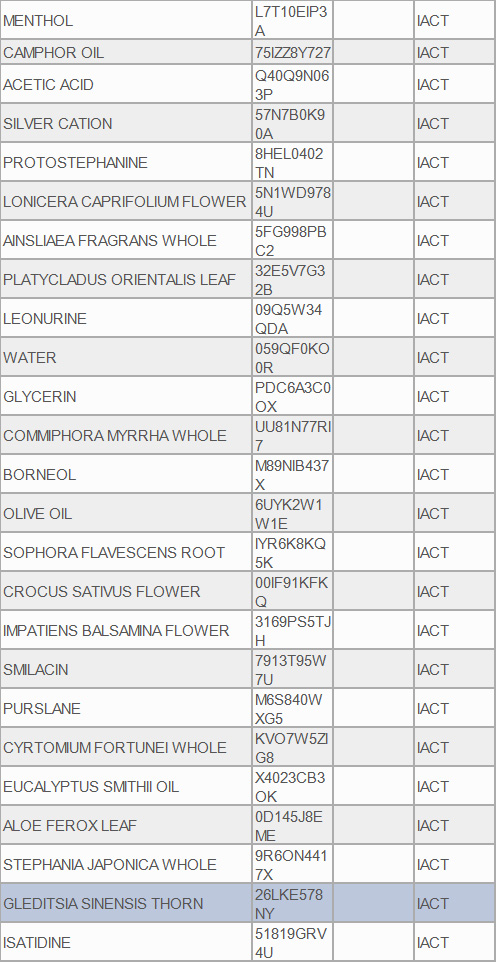
INGREDIENTS AND APPEARANCE
16BKOUTY NALL FUNGUS RENEWAL
16bkouty nall fungus renewal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84422-101 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHELLODENDRINE (UNII: AR68S526RB) (PHELLODENDRINE - UNII:AR68S526RB) PHELLODENDRINE 1 g in 100 mL UNDECYLENIC ACID (UNII: K3D86KJ24N) (UNDECYLENIC ACID - UNII:K3D86KJ24N) UNDECYLENIC ACID 10 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MENTHOL (UNII: L7T10EIP3A) CAMPHOR OIL (UNII: 75IZZ8Y727) ACETIC ACID (UNII: Q40Q9N063P) SILVER CATION (UNII: 57N7B0K90A) PROTOSTEPHANINE (UNII: 8HEL0402TN) LONICERA CAPRIFOLIUM FLOWER (UNII: 5N1WD9784U) AINSLIAEA FRAGRANS WHOLE (UNII: 5FG998PBC2) PLATYCLADUS ORIENTALIS LEAF (UNII: 32E5V7G32B) CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) 0.2 g in 100 mL LEONURINE (UNII: 09Q5W34QDA) GLYCERIN (UNII: PDC6A3C0OX) COMMIPHORA MYRRHA WHOLE (UNII: UU81N77RI7) BORNEOL (UNII: M89NIB437X) OLIVE OIL (UNII: 6UYK2W1W1E) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) CROCUS SATIVUS FLOWER (UNII: 00IF91KFKQ) IMPATIENS BALSAMINA FLOWER (UNII: 3169PS5TJH) SMILACIN (UNII: 7913T95W7U) PURSLANE (UNII: M6S840WXG5) CYRTOMIUM FORTUNEI WHOLE (UNII: KVO7W5ZIG8) EUCALYPTUS SMITHII OIL (UNII: X4023CB3OK) ALOE FEROX LEAF (UNII: 0D145J8EME) STEPHANIA JAPONICA WHOLE (UNII: 9R6ON4417X) GLEDITSIA SINENSIS THORN (UNII: 26LKE578NY) ISATIDINE (UNII: 51819GRV4U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84422-101-01 30 mL in 1 BOTTLE; Type 6: Drug/Biologic Combination 06/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/25/2024 Labeler - Shenzhen Graceful Cosmetics Co., Ltd (449897665) Registrant - Shenzhen Graceful Cosmetics Co., Ltd (449897665) Establishment Name Address ID/FEI Business Operations Shenzhen Graceful Cosmetics Co., Ltd 449897665 manufacture(84422-101) , label(84422-101)