Label: FEXOFENADINE HCL tablet, film coated
-
NDC Code(s):
69230-201-01,
69230-201-05,
69230-202-01,
69230-202-05, view more69230-202-11, 69230-202-30, 69230-202-45, 69230-202-60, 69230-202-90
- Packager: Camber Consumer Care
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
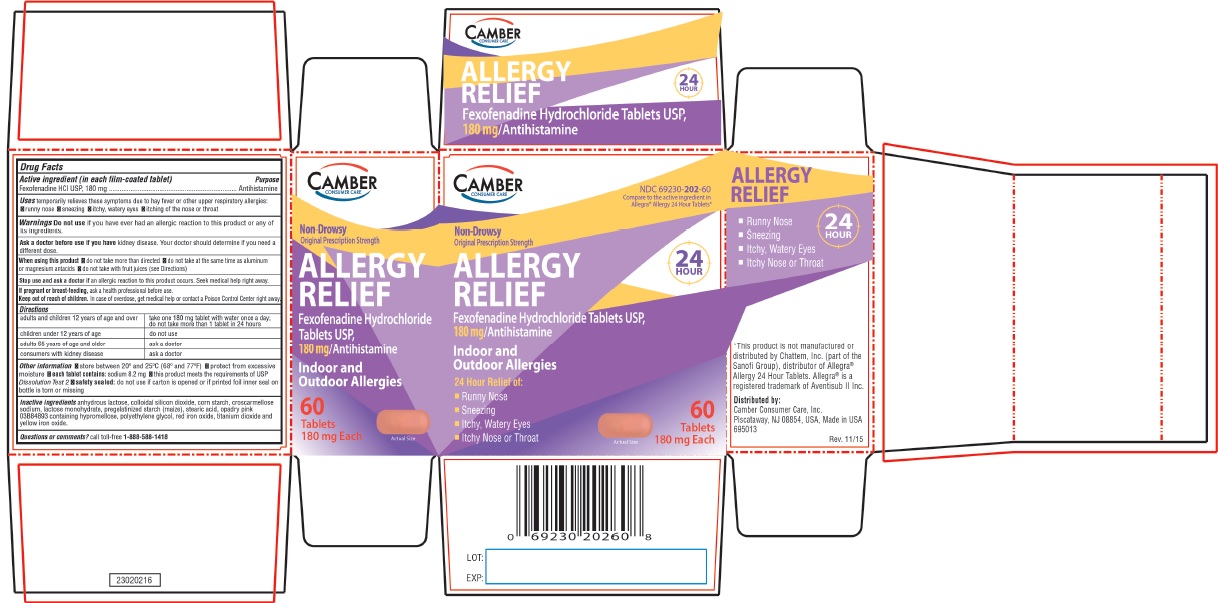
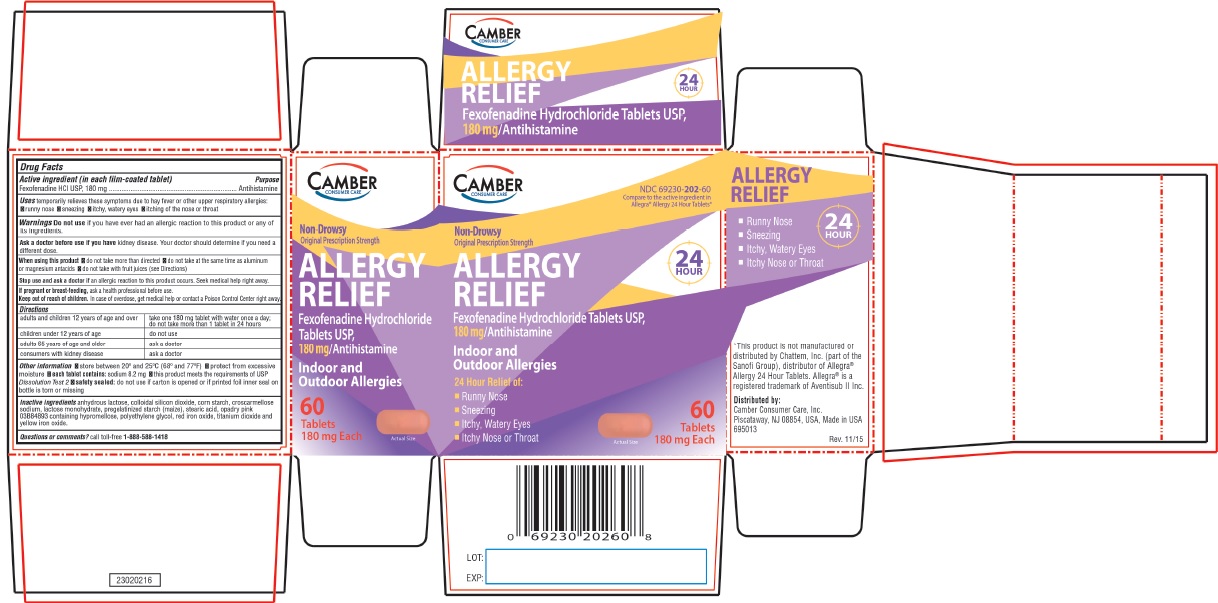
- ALLERGY Active ingredient (in each film-coated tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
-
Directions
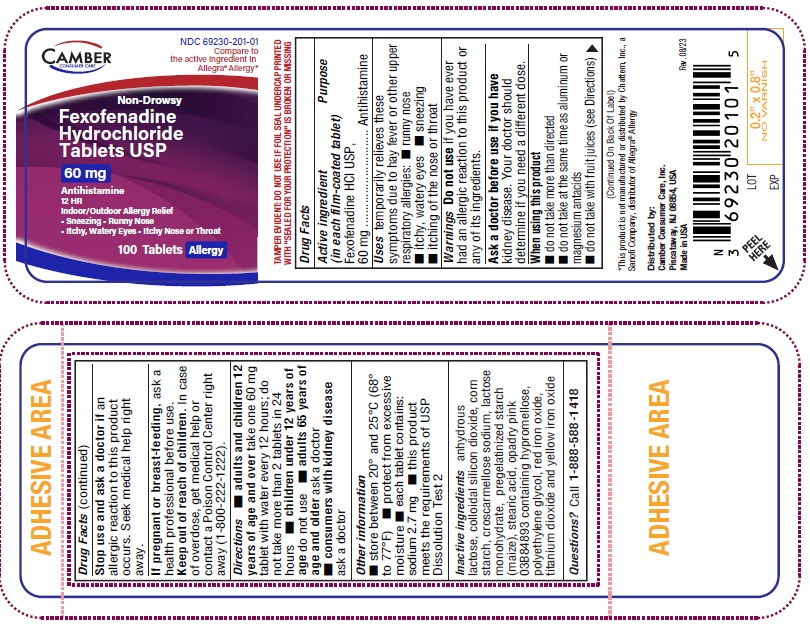
FOR 60mg adults and children 12 years of age and over take two 60mg tablets with water evrery 12 hours; do not take more than 2 tablets in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor FOR 180mg adults and children 12 years of age and over take one 180mg tablet with water once a day; do not take more than 1 tablet in 24 hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor -
Other information
- safety sealed: do not use if carton is opened or if printed foil inner seal on bottle is torn or missing
- store between 20° and 25°C (68° and 77°F)
- protect from excessive moisture
- each tablet contains: sodium 8.2 mg (for 180mg)
- each tablet contains: sodium 2.7 mg (for 60mg)
- this product meets the requirements of USP Dissolution Test 2
- Inactive ingredients
- Questions or comments?
- Camber Consumer Care - Compare to the active ingredient in Allegra® Allergy 24 Hour Tablets Allergy Relief - 24 HOUR FEXOFENADINE HYDROCHLORIDE TABLETS USP, 180 mg Antihistamine Indoor & Outdoor Allergies
-
CAMBER CONSUMER CARE
NDC 69230-201-01 Compare to the active ingredient in Allegra® Allergy*
Non-Drowsy
Fexofenadine Hydrochloride Tablets USP
60 mg
Antihistamine
12 HR Indoor/Outdoor Allergy Relief
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Nose or Throat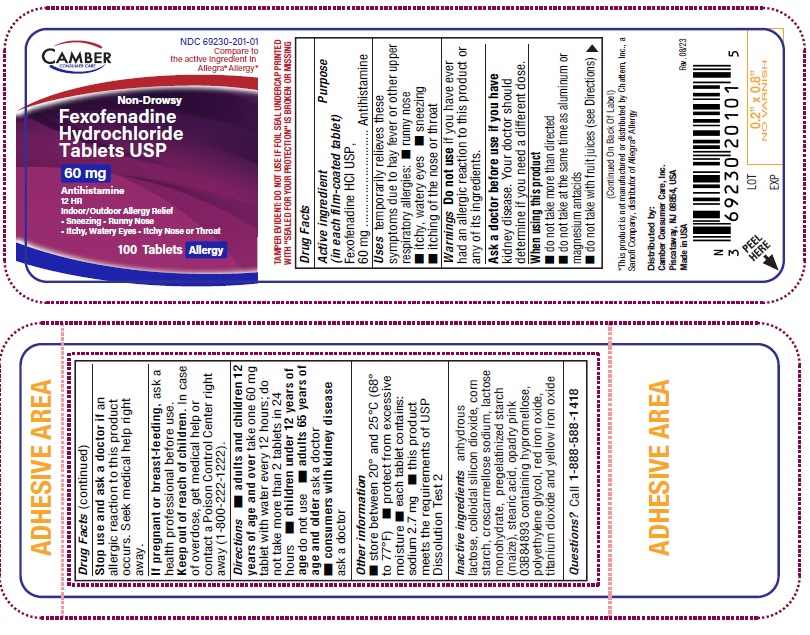
NDC 69230-201-05 Compare to the active ingredient in Allegra® Allergy*
Non-Drowsy
Fexofenadine Hydrochloride Tablets USP
60 mg
Antihistamine
12 HR Indoor/Outdoor Allergy Relief
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat

-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HCL
fexofenadine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink Score no score Shape CAPSULE Size 17mm Flavor Imprint Code SG;202 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-202-30 1 in 1 CARTON 09/16/2015 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:69230-202-45 1 in 1 CARTON 09/16/2015 2 45 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:69230-202-60 1 in 1 CARTON 09/16/2015 3 60 in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:69230-202-90 1 in 1 CARTON 09/16/2015 4 90 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:69230-202-01 1 in 1 CARTON 09/16/2015 5 100 in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:69230-202-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 09/16/2015 7 NDC:69230-202-11 1000 in 1 BOTTLE; Type 0: Not a Combination Product 09/16/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204507 09/16/2015 FEXOFENADINE HCL
fexofenadine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69230-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink Score no score Shape OVAL (Modified Oval) Size 12mm Flavor Imprint Code SG;201 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69230-201-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/08/2021 2 NDC:69230-201-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/08/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204507 12/08/2021 Labeler - Camber Consumer Care (079539968)