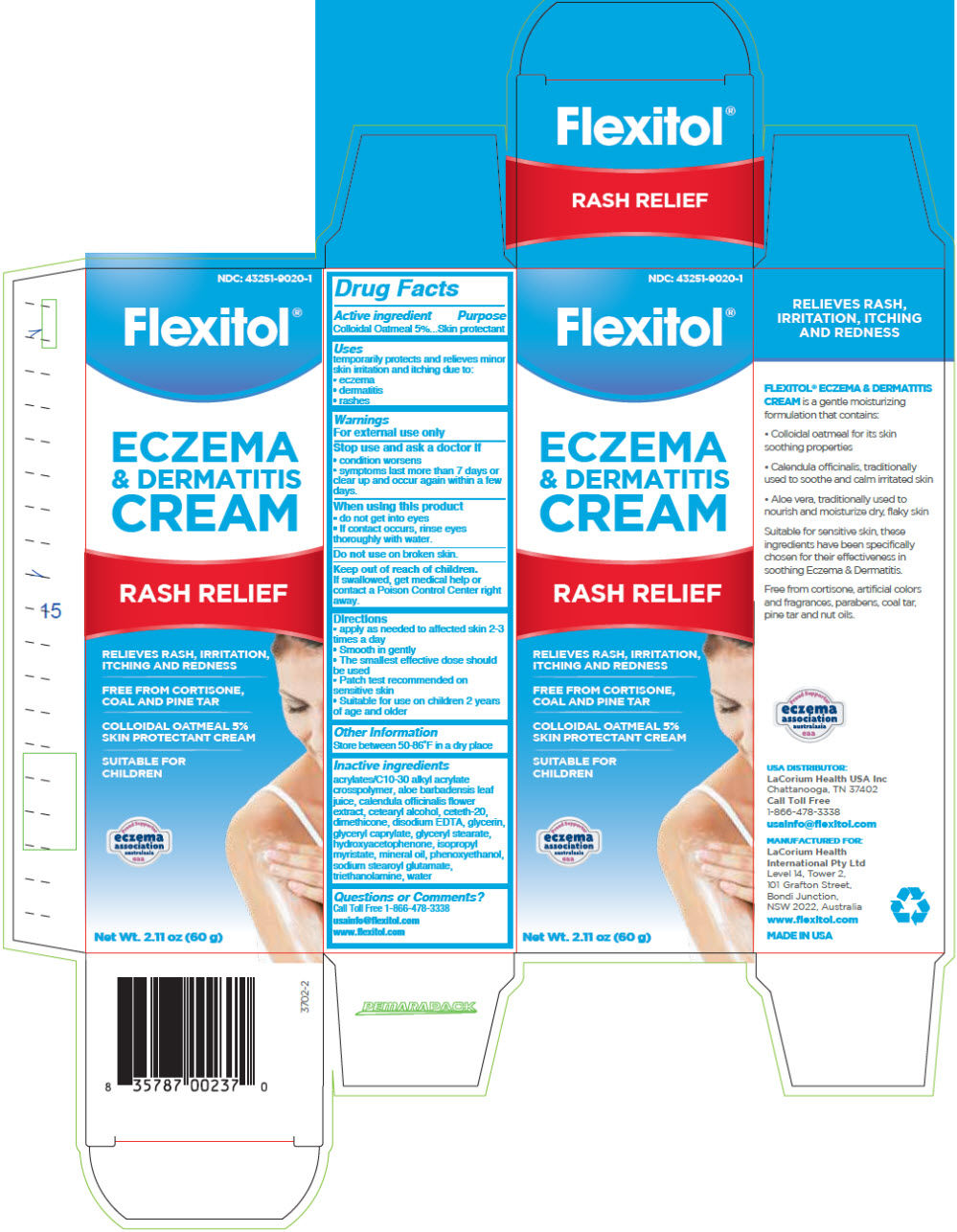
Label: FLEXITOL ECZEMA AND DERMATITIS- oatmeal cream
- NDC Code(s): 43251-9020-1, 43251-9020-2
- Packager: LaCorium Health USA Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice powder, calendula officinalis flower extract, cetearyl alcohol, ceteth-20, dimethicone 100, edetate disodium, glycerin, glyceryl caprylate, glyceryl stearate, hydroxyacetophenone, isopropyl myristate, mineral oil light, phenoxyethanol, sodium benzoate, sodium stearoyl glutamate, triethanolamine, water
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL - 60 g Tube Carton
-
INGREDIENTS AND APPEARANCE
FLEXITOL ECZEMA AND DERMATITIS
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43251-9020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength oatmeal (UNII: 8PI54V663Y) (oatmeal - UNII:8PI54V663Y) oatmeal 50 mg in 1 g Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Water (UNII: 059QF0KO0R) Carbomer Interpolymer type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Calendula Officinalis Flower (UNII: P0M7O4Y7YD) Cetostearyl Alcohol (UNII: 2DMT128M1S) Ceteth-20 (UNII: I835H2IHHX) Dimethicone (UNII: 92RU3N3Y1O) Edetate Disodium (UNII: 7FLD91C86K) Glyceryl Monocaprylate (UNII: TM2TZD4G4A) Glyceryl Monostearate (UNII: 230OU9XXE4) Glycerin (UNII: PDC6A3C0OX) Hydroxyacetophenone (UNII: G1L3HT4CMH) Isopropyl Myristate (UNII: 0RE8K4LNJS) Mineral Oil (UNII: T5L8T28FGP) Phenoxyethanol (UNII: HIE492ZZ3T) Trolamine (UNII: 9O3K93S3TK) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43251-9020-1 1 in 1 CARTON 01/10/2020 1 56 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:43251-9020-2 1 g in 1 PACKET; Type 0: Not a Combination Product 01/10/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M016 01/10/2020 Labeler - LaCorium Health USA Inc (111254392) Establishment Name Address ID/FEI Business Operations Bentley Laboratories, LLC 068351753 MANUFACTURE(43251-9020)