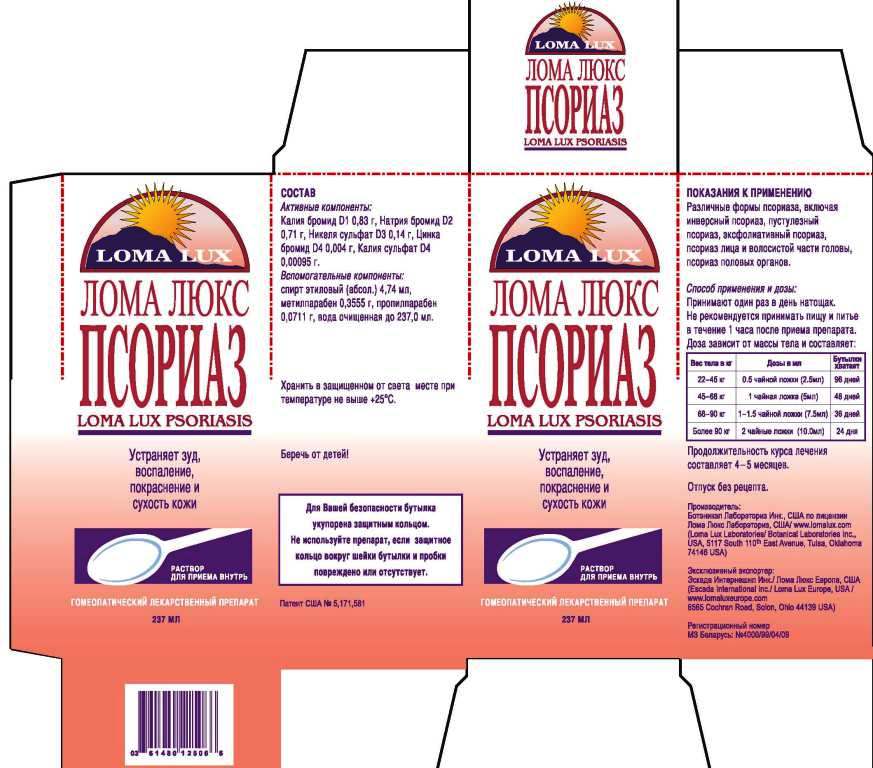
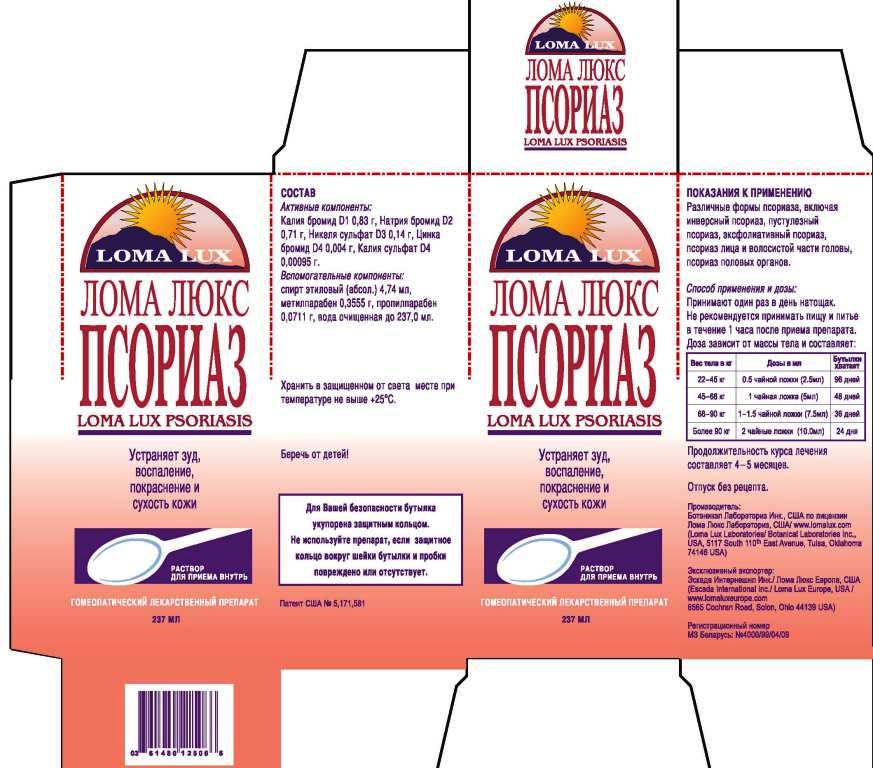
Label: LOMA LUX PSORIASIS- kali bromatum, natrum bromatum, niccolum sulphuricum, kali sulphuricum, zincum bromatum, liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57520-0713-1, 57520-0713-2, 57520-0713-3 - Packager: Apotheca Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 7, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
WARNINGS: Do not use if pregnant or nursing.
If allergic to nickel or costume jewelry, use only under the advice and supervision of a physician.
If symptoms worsen, contact a physician.
Keep out or the reach of children.
CAUTION: Use only as directed.
Do not give to children under ten years old or use in the presence of kidney disease.
If skin rash appears or if nervous symptoms persist, recur frequently or are unusual, discontinue use and consult a physician.
-
DOSAGE & ADMINISTRATION
DIRECTIONS: Take medication orally at the beginning of the day before eating or drinking anything other than water. Take nothing but water for one hour after taking medication in order to improve absorption. Continue LOMA LUX PSORIASIS as long as it is beneficial to your overall condition. Recommended minimum duration of treatment is 4-5 months.
Weight Daily Dosage Bottle Lasts
50-100 lbs 1/2 tsp 96 days
100-150 lbs 1 tsp 48 days
150-200 lbs 1 1/2 tsp 36 days
Over 200 lbs 2 tsp 24 days
- INACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LOMA LUX PSORIASIS
kali bromatum, natrum bromatum, niccolum sulphuricum, kali sulphuricum, zincum bromatum, liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57520-0713 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM BROMIDE (UNII: OSD78555ZM) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM BROMIDE 1 [hp_X] in 1 mL SODIUM BROMIDE (UNII: LC1V549NOM) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM BROMIDE 2 [hp_X] in 1 mL NICKEL SULFATE HEXAHYDRATE (UNII: JC9WZ4FK68) (NICKEL - UNII:7OV03QG267) NICKEL SULFATE HEXAHYDRATE 3 [hp_X] in 1 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM SULFATE 4 [hp_X] in 1 mL ZINC BROMIDE (UNII: OO7ZBU9703) (ZINC BROMIDE - UNII:OO7ZBU9703) ZINC BROMIDE 4 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57520-0713-3 1 in 1 CARTON 1 NDC:57520-0713-2 1 in 1 CARTON 1 NDC:57520-0713-1 237 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/07/2011 Labeler - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture