Label: IONOSOL MB AND DEXTROSE- dextrose monohydrate, sodium lactate, potassium chloride, magnesium chloride, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate injection, solution
- NDC Code(s): 0990-7372-03, 0990-7372-62
- Packager: ICU Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ionosol MB and 5% Dextrose Injection (Multiple Electrolytes and 5% Dextrose Injection Type 1, USP) is a sterile, nonpyrogenic solution designed for intravenous administration. The solution is formulated to provide fluid and electrolytes for treatment of dehydration and acidosis.
Each 100 mL contains dextrose, hydrous 5 g; sodium lactate, anhydrous 260 mg; potassium chloride 141 mg; magnesium chloride, hexahydrate 30 mg; monobasic potassium phosphate, anhydrous 15 mg; and monobasic sodium phosphate, monohydrate 25 mg.
Each liter contains 25 mEq sodium (Na+); 20 mEq potassium (K+); 3 mEq magnesium (Mg++); 22 mEq chloride (Cl¯); 3 mM of phosphate (PO4≡); and 23 mEq lactate (CH3CH(OH)COO¯).
The electrolyte content is hypotonic (100 mOsmol/L) in relation to the extracellular fluid (1pprox.. 280 mOsmol/L). The osmolarity for the total solution is 352 mOsmol/L (calc.). May contain hydrochloric acid for pH adjustment. pH is 5.0 (4.0 to 6.5).
Dextrose, USP, hydrous is chemically designated C6H12O6 • H2O (D-glucose, monohydrate), a hexose sugar freely soluble in water. Dextrose, hydrous has the following structural formula:
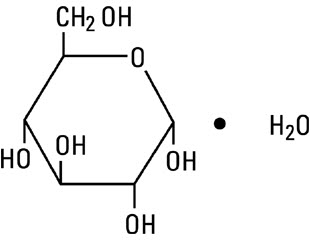
Magnesium Chloride, USP, hexahydrate is chemically designated MgCl2 • 6H2O, colorless flakes or crystals very soluble in water.
Potassium Chloride, USP is chemically designated KCl, a white granular powder freely soluble in water.
Monobasic Potassium Phosphate, NF, anhydrous is chemically designated KH2PO4, colorless crystals or white granular powder freely soluble in water.
Sodium Lactate, USP is chemically designated CH3CH(OH)COONa, a 60% aqueous solution miscible in water.
Monobasic Sodium Phosphate, USP, monohydrate is chemically designated NaH2PO4• H2O, white crystals or granules freely soluble in water.
Water for Injection, USP is chemically designated H2O.
The flexible plastic container is fabricated from a specially formulated polyvinylchloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions inside the plastic container also can leach out certain of their chemical components in very small amounts before the expiration period is attained. However, the safety of the plastic has been confirmed by tests in animals according to USP biological standards for plastic containers.
-
CLINICAL PHARMACOLOGY
Ionosol MB and 5% Dextrose Injection contains a hypotonic concentration of electrolytes with dextrose. The letters "MB" mean "modified Butler's" solution; the modified solution contains 5 mEq less sodium, 5 mEq more potassium, and 3 mEq added magnesium, as compared with the original Butler's solution. The modified solution can be used in pediatric patients for treatment of dehydration, acidosis, diarrhea, or burns, and in adults for postoperative fluid and electrolyte therapy.
Solutions containing dextrose restore blood glucose levels and provide calories. Carbohydrate in the form of dextrose may aid in minimizing liver glycogen depletion and exerts a protein-sparing action. Dextrose injected parenterally undergoes oxidation to carbon dioxide and water.
The lactate anion provides an alkalizing effect resulting from simultaneous removal by the liver of lactate and hydrogen ions. In the liver, the lactate is metabolized to glycogen which is ultimately converted to carbon dioxide and water by oxidative metabolism.
The lactate anion acts as a source (alternate) of bicarbonate when normal production and utilization of lactic acid is not impaired as a result of disordered lactate metabolism. Since metabolic conversion is dependent on the integrity of cellular oxidative processes, lactate may be inadequate or ineffective as a source of bicarbonate in patients suffering from acidosis associated with shock or other disorders involving reduced perfusion of body tissues. When oxidative activity is intact, one to two hours time is required for metabolism of lactate.
Magnesium chloride in water dissociates to provide magnesium (Mg++) and chloride (Cl‾) ions. Magnesium is the second most plentiful cation of the intracellular fluids. It is an important cofactor for enzymatic reactions and plays an important role in neurochemical transmission and muscular excitability. Normal plasma concentration ranges from 1.5 to 2.5 or 3.0 mEq/liter. Magnesium is excreted solely by the kidney at a rate proportional to the plasma concentration and glomerular filtration.
Phosphate is one of the three major intracellular electrolytes (along with potassium and magnesium) and the largest anion component found within the cells. Its concentration and excretion are largely dependent on intake, acid-base balance and endocrine function. Its metabolism follows that of calcium in many respects. Phosphate anion in electrolyte solutions may help to repair phosphate deficiency.
Potassium chloride in water dissociates to provide potassium (K+) and chloride (Cl‾) ions. Potassium is the chief cation of body cells (160 mEq/liter of intracellular water). It is found in low concentration in plasma and extracellular fluids (3.5 to 5.0 mEq/liter in a healthy adult and child over 10 days old; 3.5 to 6.0 mEq/liter in a child less than 10 days old). Potassium plays an important role in electrolyte balance. Normally about 80 to 90% of the potassium intake is excreted in the urine; the remainder in the stools and to a small extent, in the perspiration. The kidney does not conserve potassium well so that during fasting or in patients on a potassium-free diet, potassium loss from the body continues resulting in potassium depletion.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirement ranges from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production). Average normal pediatric daily requirements are based on the child's weight as described in the table below:
Weight
Fluid Requirements
Up to 10 kg
100 mL/kg
11 to 20 kg
1,000 mL + 50 mL/kg for
each kg above 10 kg
Above 20 kg
1,500 mL + 20 mL/kg for
each kg above 20 kg
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments, and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
Ionosol MB and 5% Dextrose Injection contains a hypotonic electrolyte concentration. This should not be confused with the total tonicity (electrolytes plus nonelectrolytes) of solutions containing both electrolytes and dextrose. In general, solutions providing isotonic electrolyte concentrations are most applicable to replacement of acute deficits, whereas hypotonic electrolyte concentrations are best suited for parenteral maintenance of water requirements when only small quantities of electrolytes are desired.
-
INDICATIONS AND USAGE
Ionosol MB and 5% Dextrose Injection is indicated for intravenous administration to infants for treatment of dehydration, acidosis, diarrhea, and burns, but only after administration of an initial priming solution: 15 mL of 5% dextrose and 0.45% Sodium Chloride Injection/kg of body weight.
In adults, Ionosol MB and 5% Dextrose Injection is indicated postoperatively for intravenous fluid and electrolyte maintenance therapy, with a small amount of carbohydrate calories for reducing catabolism of endogenous protein reserves.
- CONTRAINDICATIONS
-
WARNINGS
Solutions which contain potassium ions should be used with great care, if at all, in patients with hyperkalemia, severe renal failure and in conditions in which potassium retention is present.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
In patients with diminished renal function, administration of solutions containing sodium or potassium ions may result in sodium or potassium retention.
Solutions containing lactate ions should be used with great care, if at all, in patients with metabolic or respiratory alkalosis. The administration of lactate ions should be done with great care in those conditions in which there is an increased level or an impaired utilization of lactate ions, such as severe hepatic insufficiency.
The intravenous administration of Ionosol MB and 5% Dextrose Injection can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutional states is inversely proportional to the electrolyte concentrations of administered parenteral solutions. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of such solutions.
-
PRECAUTIONS
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
Caution must be exercised in the administration of parenteral fluids, especially those containing sodium ions, to patients receiving corticosteroids or corticotropin.
Solutions containing lactate ions should be used with caution as excess administration may result in metabolic alkalosis.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
Pregnancy Category C.
Animal reproduction studies have not been conducted with Ionosol solutions. It is also not known whether Ionosol solutions can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Ionosol solutions should be given to a pregnant woman only if clearly needed.
Geriatric Use:
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Pediatric Use:
The safety and effectiveness in the pediatric population are based on the similarity of the clinical conditions of the pediatric and adult populations. In neonates or very small infants the volume of fluid may affect fluid and electrolyte balance.
Frequent monitoring of serum glucose concentrations is required when dextrose is prescribed to pediatric patients, particularly neonates and low birth weight infants.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
The dose is dependent upon the age, weight and clinical condition of the patient.
In infants, Ionosol MB and 5% Dextrose Injection is given only after administration of an initial priming solution: 15 mL of half isotonic saline in 5% dextrose and 0.45% Sodium Chloride Injection/kg of body weight, administered to small infants at a maximum rate of 0.8 mL/minute. Infants typically tolerate not more than 150 to 200 mL of Ionosol MB and 5% Dextrose Injection per kg body weight/day.
As reported in the literature, the dosage and constant infusion rate of intravenous dextrose must be selected with caution in pediatric patients, particularly neonates and low birth weight infants, because of the increased risk of hyperglycemia/hypoglycemia.
In adults, intravenous infusions of Ionosol MB and 5% Dextrose Injection are given postoperatively, at a rate not greater than 4 mL/minute.
Drug Interactions
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. See PRECAUTIONS.
INSTRUCTIONS FOR USE
To Open:
Tear outer wrap at notch and remove solution container. Some opacity of the plastic due to moisture absorption during sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
If supplemental medication is desired, follow directions below before preparing for administration.
To Add Medication
-
Prepare additive port.
-
Using aseptic technique and an additive delivery needle of appropriate length, puncture resealable additive port at target area, inner diaphragm and inject. Withdraw needle after injecting medication.
-
The additive port may be protected by covering with an additive cap.
-
Mix container contents thoroughly.
To Administer
-
Attach administration set per manufacturer's instructions.
-
Regulate rate of administration per institutional policy.
WARNING: Do not use flexible container in series connections.
-
-
HOW SUPPLIED
Ionosol MB and 5% Dextrose Injection (Multiple Electrolytes and 5% Dextrose Injection Type 1, USP) is supplied in flexible plastic single-dose containers as follows:
NDC Fill Volume/Container size (mL) ICU Medical is transitioning NDC codes from the "0409" to a "0990" labeler code. Both NDC codes are expected to be in the market for a period of time. 0409-7372-62* 250/250 0990-7372-62* 250/250 0409-7372-03† 500/500 0990-7372-03*,† 500/500 Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: August, 2020
ICU Medical, Inc. Lake Forest, Illinois, 60045, USA
IFU0000258 -
PRINCIPAL DISPLAY PANEL - 500 mL Bag Label
500 mL
NDC 0990-7372-03IONOSOL® MB and
5% DEXTROSE INJECTION
(MULTIPLE ELECTROLYTES AND 5% DEXTROSE INJECTION TYPE 1, USP)A MAINTENANCE ELECTROLYTE SOLUTION
EACH 100 mL CONTAINS DEXTROSE, HYDROUS 5 g; MAGNESIUM
CHLORIDE, HEXAHYDRATE 30 mg; POTASSIUM CHLORIDE 141 mg;
MONOBASIC POTASSIUM PHOSPHATE, ANHYDROUS 15 mg; MONOBASIC
SODIUM PHOSPHATE, MONOHYDRATE 25 mg; SODIUM LACTATE,
ANHYDROUS 260 mg.ELECTROLYTES PER LITER (NOT INCLUDING IONS FOR pH ADJUSTMENT):
SODIUM 25 mEq; POTASSIUM 20 mEq; MAGNESIUM 3 mEq; CHLORIDE 22
mEq; PHOSPHATE 3 mM; LACTATE 23 mEq. MAY CONTAIN HYDROCHLORIC
ACID FOR pH ADJUSTMENT. STERILE, NONPYROGENIC.352 mOsmol/LITER (CALC.)
pH 5.0 (4.0 to 6.5)ADDITIVES MAY BE INCOMPATIBLE.
CONSULT WITH PHARMACIST, IF AVAILABLE.
WHEN INTRODUCING ADDITIVES, USE ASEPTIC TECHNIQUE,
MIX THOROUGHLY AND DO NOT STORE.SINGLE-DOSE CONTAINER. FOR INTRAVENOUS USE.
USUAL DOSAGE: SEE INSERT. MUST
NOT BE USED IN SERIES CONNECTIONS.3
Vicumedical
Rx ONLY
CONTAINS DEHPIM-4445
ICU Medical, Inc., Lake Forest, Illinois, 60045, USA
-
PRINCIPAL DISPLAY PANEL - Overwrap Label
TO OPEN TEAR AT NOTCH
2
HDPEDO NOT REMOVE FROM OVERWRAP UNTIL READY FOR USE. AFTER REMOVING
THE OVERWRAP, CHECK FOR MINUTE LEAKS BY SQUEEZING CONTAINER FIRMLY.
IF LEAKS ARE FOUND, DISCARD SOLUTION AS STERILITY MAY BE IMPAIRED.
RECOMMENDED STORAGE: ROOM TEMPERATURE (25°C). AVOID EXCESSIVE
HEAT. PROTECT FROM FREEZING. SEE INSERT.
98-4321-R14-3/98
-
INGREDIENTS AND APPEARANCE
IONOSOL MB AND DEXTROSE
dextrose monohydrate, sodium lactate, potassium chloride, magnesium chloride, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0990-7372 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 5 g in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 260 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 141 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 30 mg in 100 mL MONOBASIC POTASSIUM PHOSPHATE (UNII: 4J9FJ0HL51) (POTASSIUM CATION - UNII:295O53K152, PHOSPHATE ION - UNII:NK08V8K8HR) MONOBASIC POTASSIUM PHOSPHATE 15 mg in 100 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (SODIUM CATION - UNII:LYR4M0NH37, PHOSPHATE ION - UNII:NK08V8K8HR) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 25 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0990-7372-62 24 in 1 CASE 07/01/2019 1 1 in 1 POUCH 1 250 mL in 1 BAG; Type 0: Not a Combination Product 2 NDC:0990-7372-03 24 in 1 CASE 01/01/2020 2 1 in 1 POUCH 2 500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019513 07/01/2019 Labeler - ICU Medical Inc. (118380146)


