Label: OWELL NATURALS DRAW SALVE- arnica montana hpus, silicea hpus salve
- NDC Code(s): 83570-487-02
- Packager: Owell Naturals Brand LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
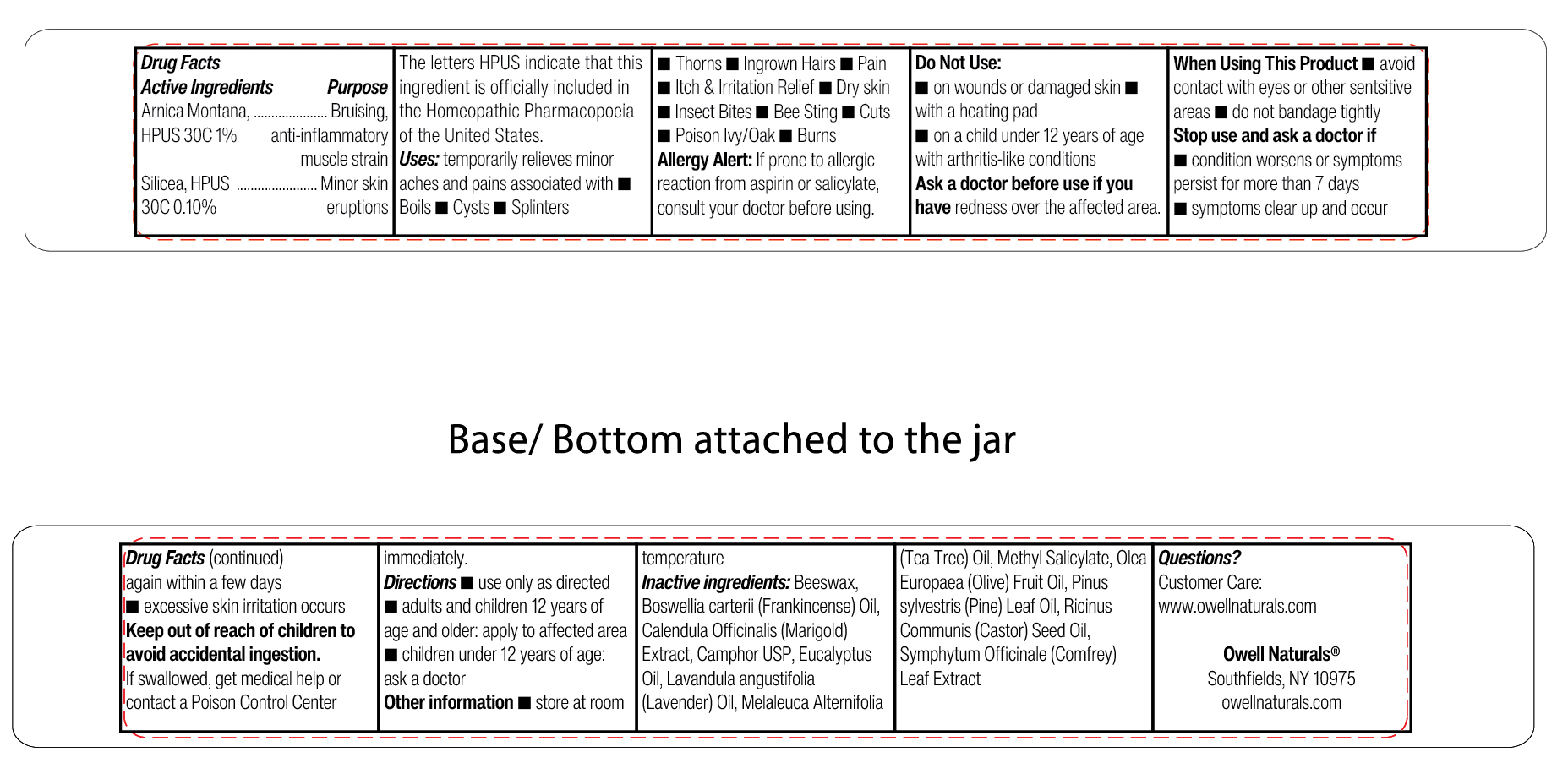
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- QUESTIONS
-
INACTIVE INGREDIENT
Inactive Ingredients
Beeswax, Boswellia carterii (Frankincense) Oil, Calendula Officinalis (Marigold) Extract, Camphor USP, Eucalyptus Oil,
Lavandula angustifolia (Lavender) Oil, Melaleuca alternifolia (Tea Tree) Oil, Methyl Salicylate, Olea Europaea (Olive) Fruit Oil,
Pinus sylvestris (Pine) Leaf Oil, Ricinus communis (Castor) Seed Oil, Symphytum Officinale (Comfrey) Leaf Extract
- PURPOSE
-
WARNINGS
Allergy Alert: If prone to allergic reaction from aspirin or salicylate, consult your doctor before using.
Do Not Use:
on wounds or damaged skin
with a heating pad
on a child under 12 years of age with arthritis-like conditions
Stop use and ask a doctor if
condition worsens or symptoms persist for more than 7 days
symptoms clear up and occur again within a few days
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OWELL NATURALS DRAW SALVE
arnica montana hpus, silicea hpus salveProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83570-487 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 0.283 g in 28.3 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 0.0283 g in 28.3 g Inactive Ingredients Ingredient Name Strength WHITE WAX (UNII: 7G1J5DA97F) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) LAVENDER OIL (UNII: ZBP1YXW0H8) TEA TREE OIL (UNII: VIF565UC2G) WHITE PINE OIL (UNII: HA5CX6676U) EUCALYPTUS OIL (UNII: 2R04ONI662) OLIVE OIL (UNII: 6UYK2W1W1E) CASTOR OIL (UNII: D5340Y2I9G) FRANKINCENSE OIL (UNII: 67ZYA5T02K) COMFREY LEAF (UNII: DG4F8T839X) METHYL SALICYLATE (UNII: LAV5U5022Y) CAMPHOR (NATURAL) (UNII: N20HL7Q941) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83570-487-02 56 g in 1 JAR; Type 0: Not a Combination Product 06/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/25/2024 Labeler - Owell Naturals Brand LLC (122280778)