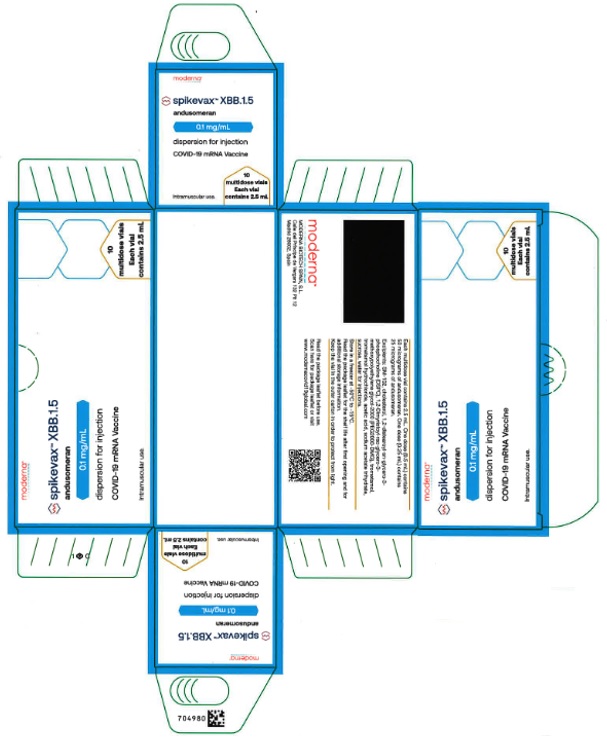
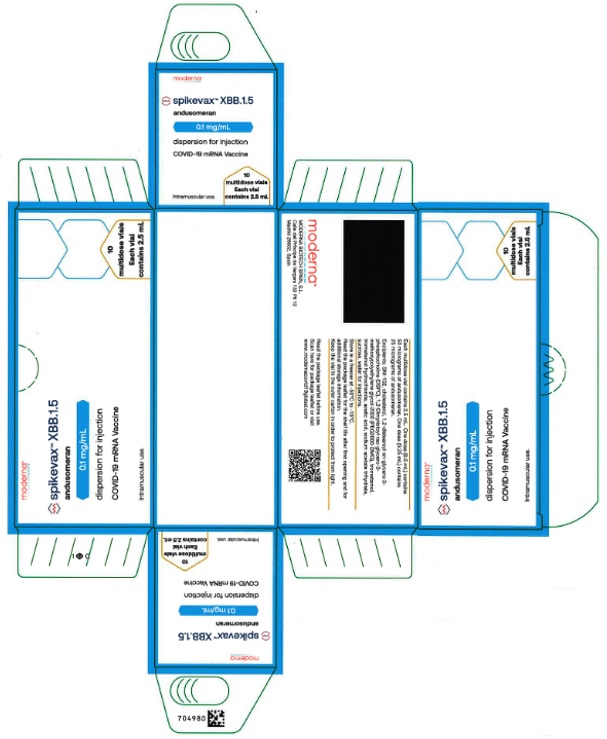
Label: SPIKEVAX- covid-19 vaccine, mrna injection, solution
- NDC Code(s): 61434-061-00, 61434-061-01
- Packager: Catalent Indiana, LLC
- Category: VACCINE LABEL
Drug Label Information
Updated December 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SPIKEVAX
covid-19 vaccine, mrna injection, solutionProduct Information Product Type VACCINE Item Code (Source) NDC:61434-061 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CX-038839 OMICRON (XBB.1.5) (UNII: 4F9QRS7ZV2) (CX-038839 OMICRON (XBB.1.5) - UNII:4F9QRS7ZV2) CX-038839 OMICRON (XBB.1.5) 50 ug in 0.5 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61434-061-01 10 in 1 CARTON 1 NDC:61434-061-00 2.5 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date export only 09/11/2023 Labeler - Catalent Indiana, LLC (172209277)