Label: PREMIER VALUE- dextromethorphan hbr, guaifenesin solution
- NDC Code(s): 68016-744-04
- Packager: PHARMACY VALUE ALLIANCE LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 6, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
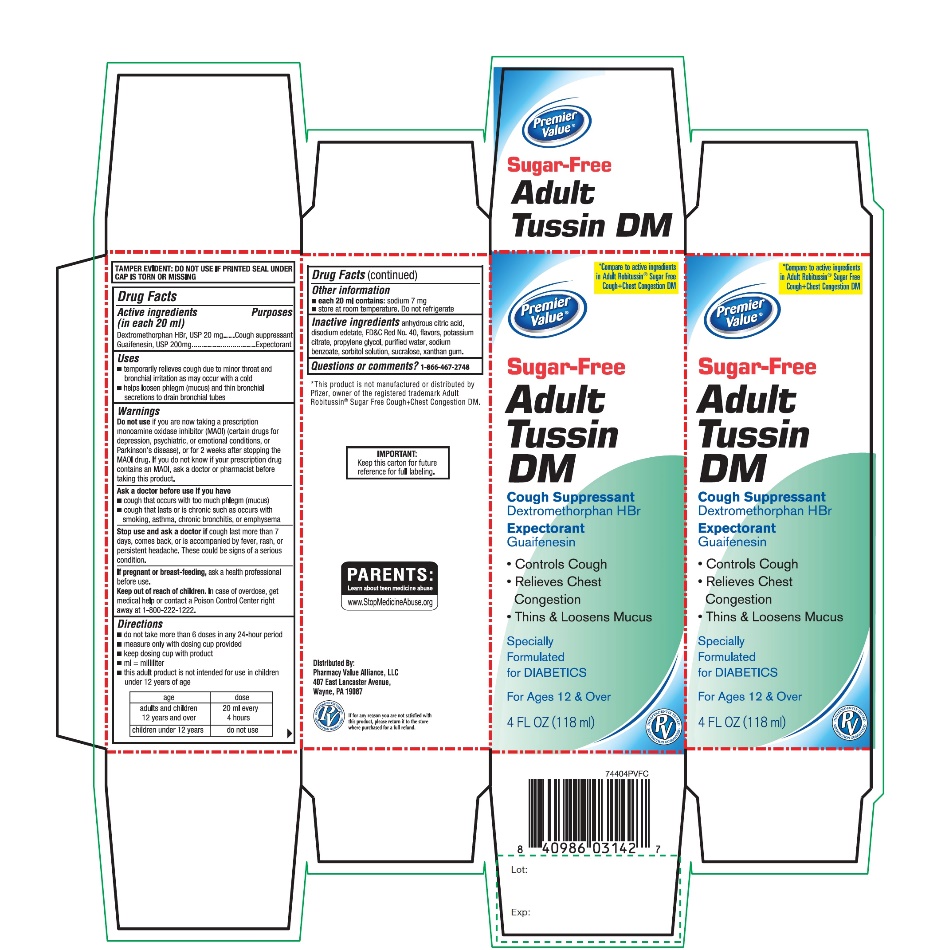
- Active ingredients (in each 20 mL)
- Purposes
- Uses
- Warnings
-
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- ▪
- shake well before using
- ▪
- do not take more than 6 doses in any 24-hour period
- ▪
- measure only with dosing cup provided
- ▪
- keep dosing cup with product
- ▪
- mL = milliliter
- ▪
- this adult product is not intended for use in children under 12 years of age
age
dose
adults and children 12 years and over
20 mL every 4 hours
children under 12 years
do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
Compare to the active ingredients of Robitussin® Sugar Free Cough + Congestion DM
NDC# 68016-744-04
Premier Value®
Sugar Free
Tussin DM
Cough Suppressant
Dextromethorphan HBr
Expectorant
Guaifenesin
- •
- Relieves Chest Congestion
- •
- Controls cough and mucus
Specially Formulated for DIABETICS
Non-Drowsy
For Ages 12 & Over
4 FL OZ (118 mL)
IMPORTANT: Keep this carton for future reference on full labeling.
Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
*This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin® Sugar Free Cough + Congestion DM.
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE
dextromethorphan hbr, guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-744 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color RED Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-744-04 1 in 1 CARTON 03/20/2019 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 03/20/2019 Labeler - PHARMACY VALUE ALLIANCE LLC (101668460)