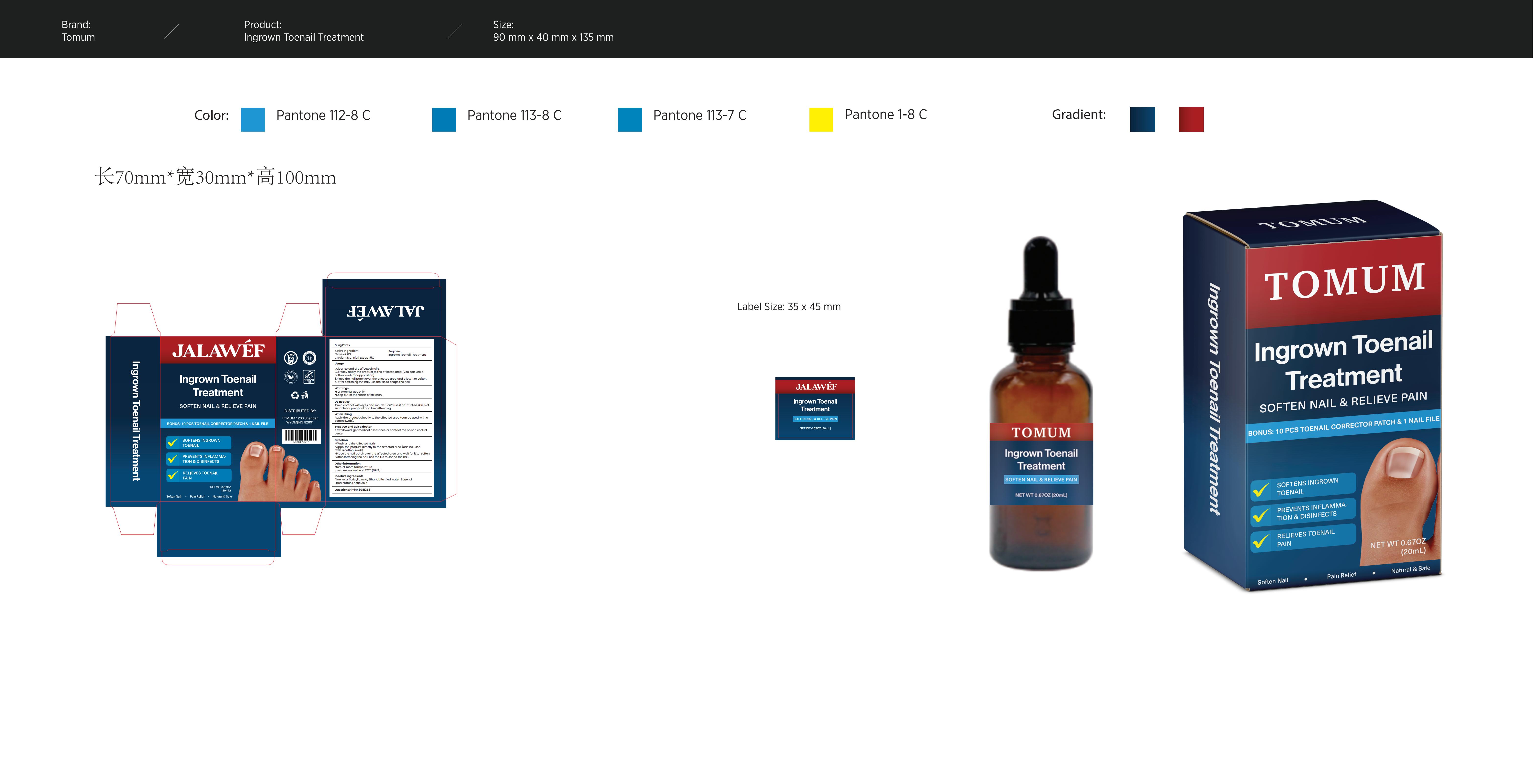
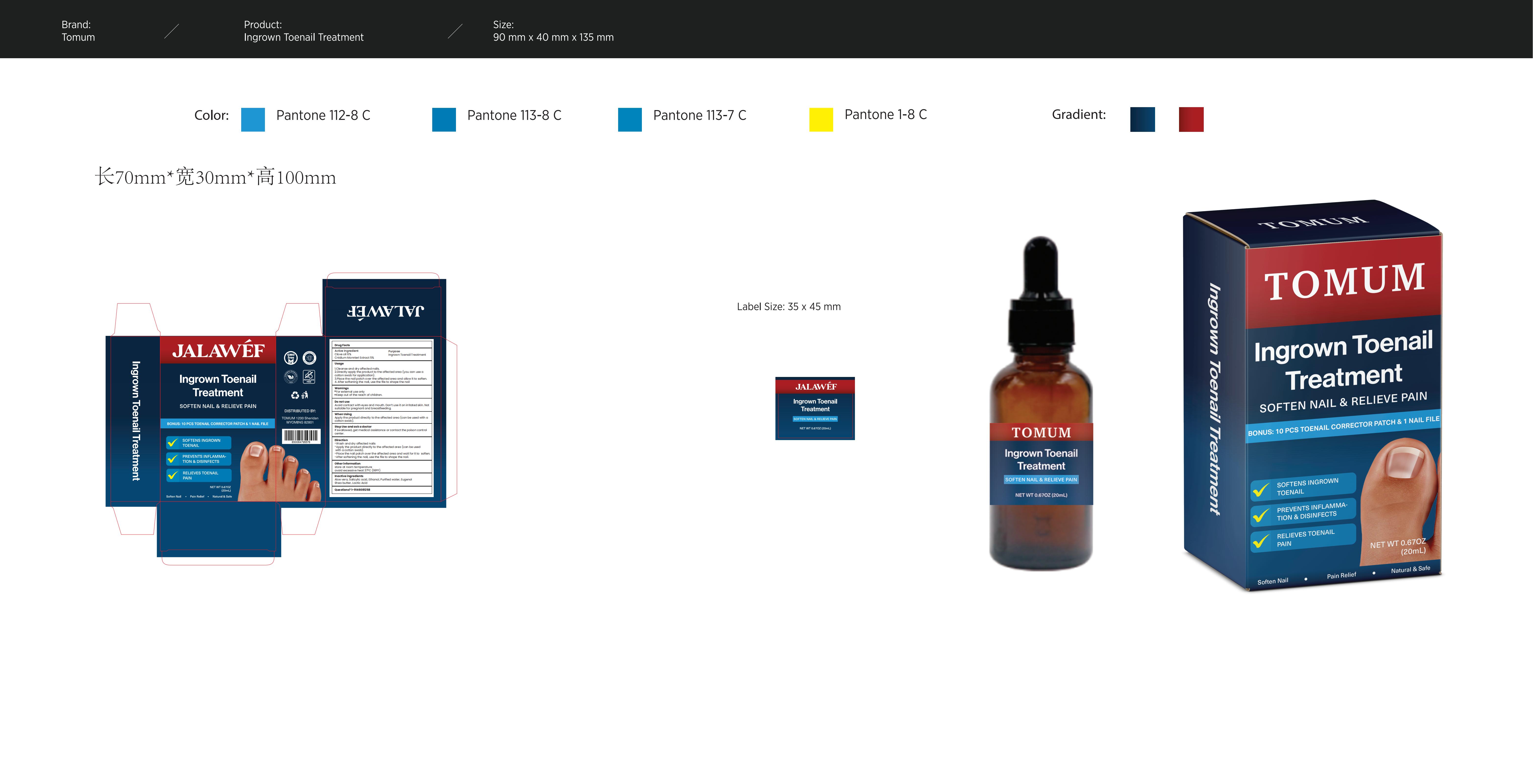
Label: INGROWN NAIL TREATMENT- jalawef ingrown nail treatment liquid
- NDC Code(s): 83299-029-01
- Packager: Consilii LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INGROWN NAIL TREATMENT
jalawef ingrown nail treatment liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83299-029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) (CNIDIUM MONNIERI FRUIT - UNII:V1IA3S3CUS) CNIDIUM MONNIERI FRUIT 5 g in 100 mL CLOVE OIL (UNII: 578389D6D0) (CLOVE OIL - UNII:578389D6D0) CLOVE OIL 6 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) SALICYLIC ACID (UNII: O414PZ4LPZ) WATER (UNII: 059QF0KO0R) SHEA BUTTER (UNII: K49155WL9Y) ALCOHOL (UNII: 3K9958V90M) EUGENOL (UNII: 3T8H1794QW) LACTIC ACID (UNII: 33X04XA5AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83299-029-01 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/13/2024 Labeler - Consilii LLC (118891890) Establishment Name Address ID/FEI Business Operations Consilii LLC 118891890 label(83299-029) , manufacture(83299-029)