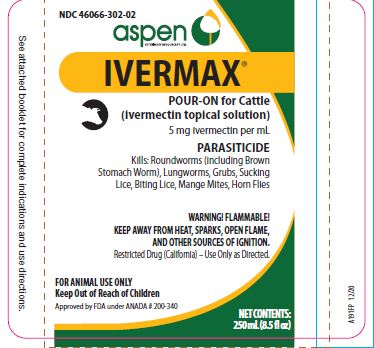
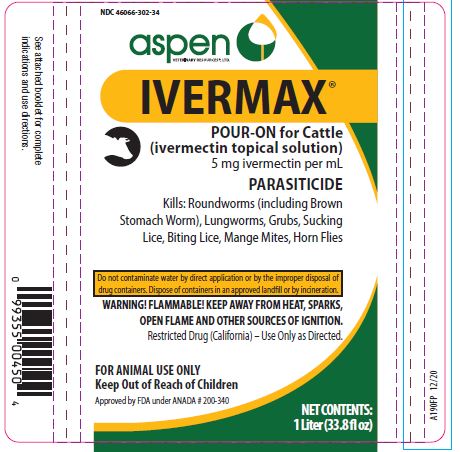
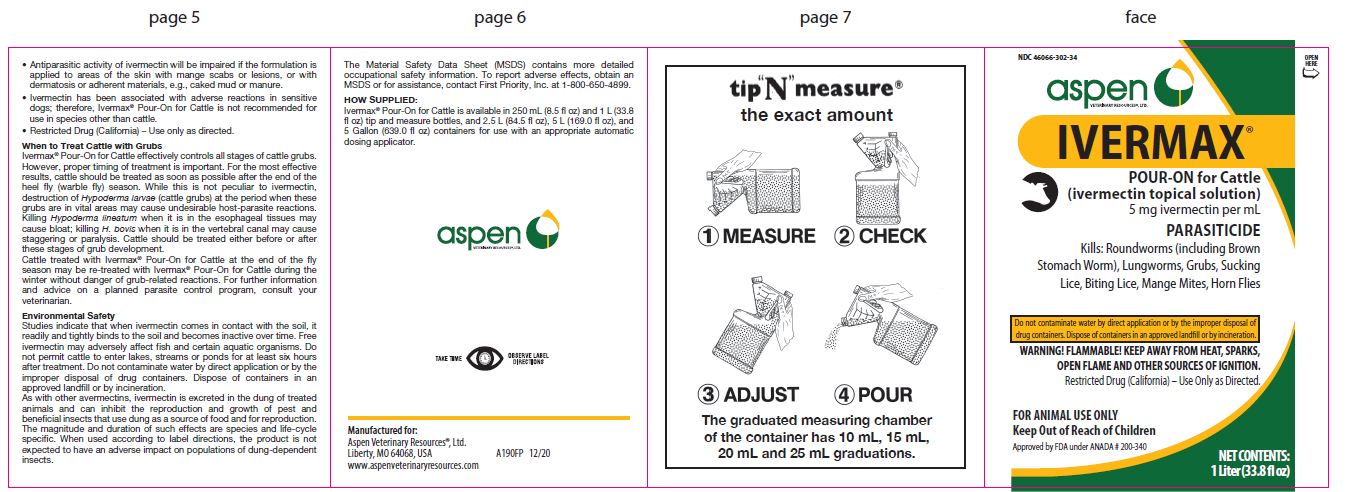
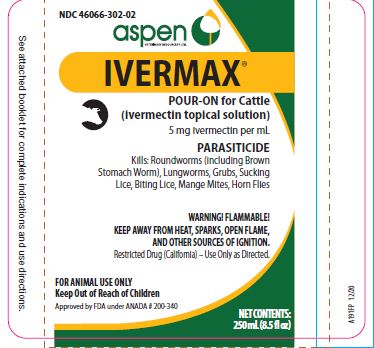
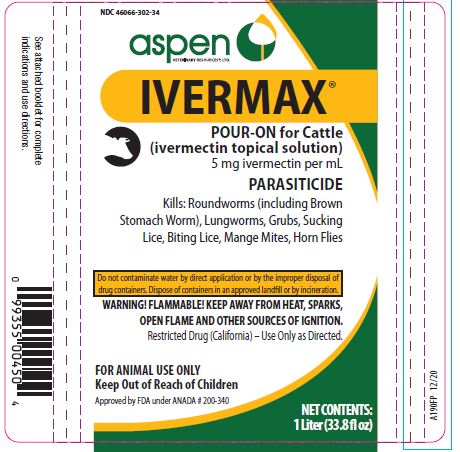
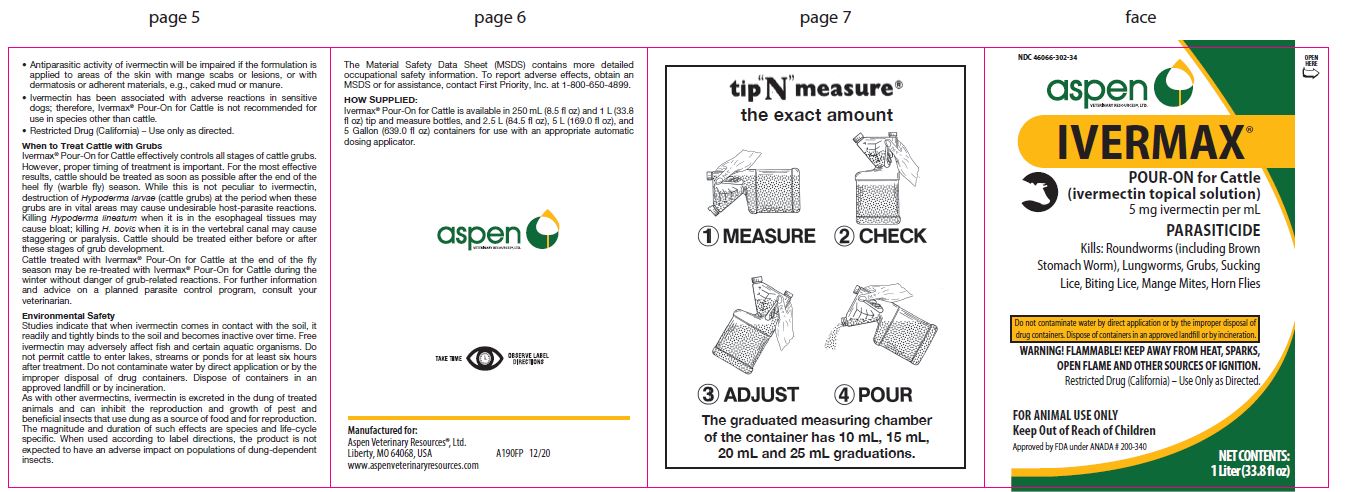
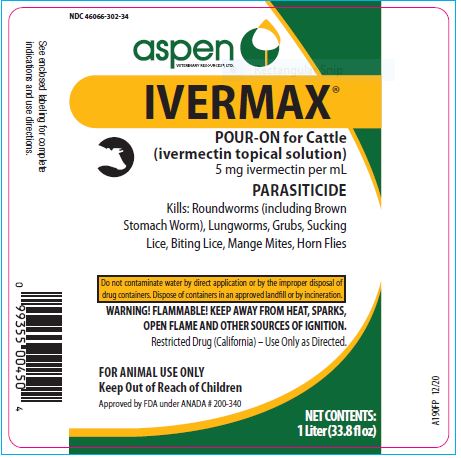
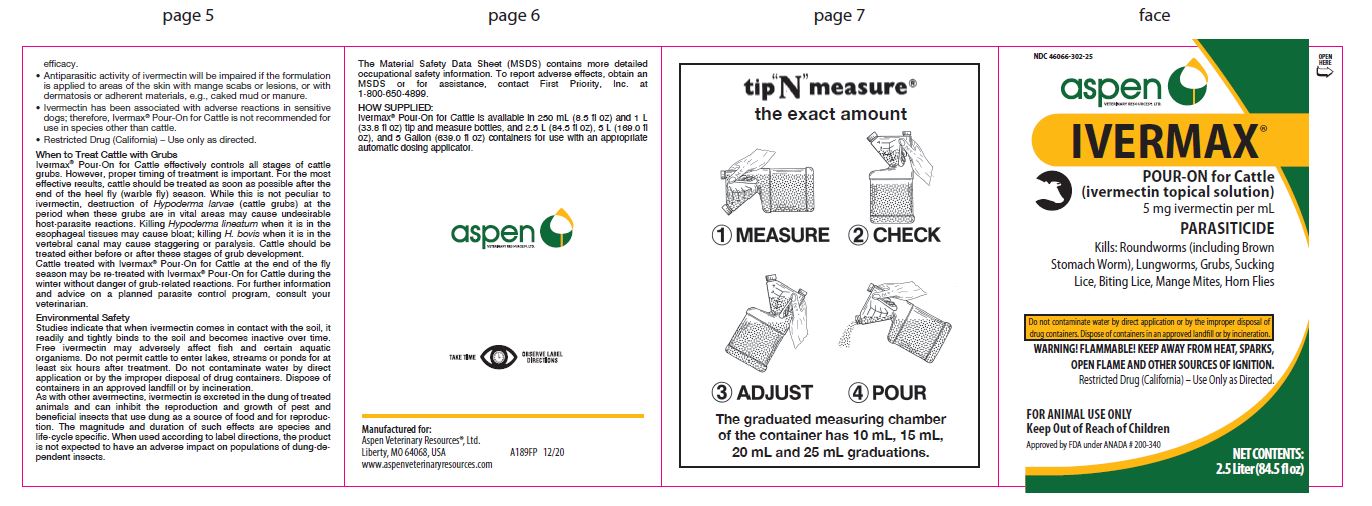
Label: IVERMAX POUR-ON FOR CATTLE- ivermectin cattle pour-on solution
- NDC Code(s): 46066-302-02, 46066-302-25, 46066-302-34
- Packager: ASPEN VETERINARY RESOURCES, LTD.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated January 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INFORMATION FOR OWNERS/CAREGIVERS
- Parasiticide
- Introduction
-
Mode of Action
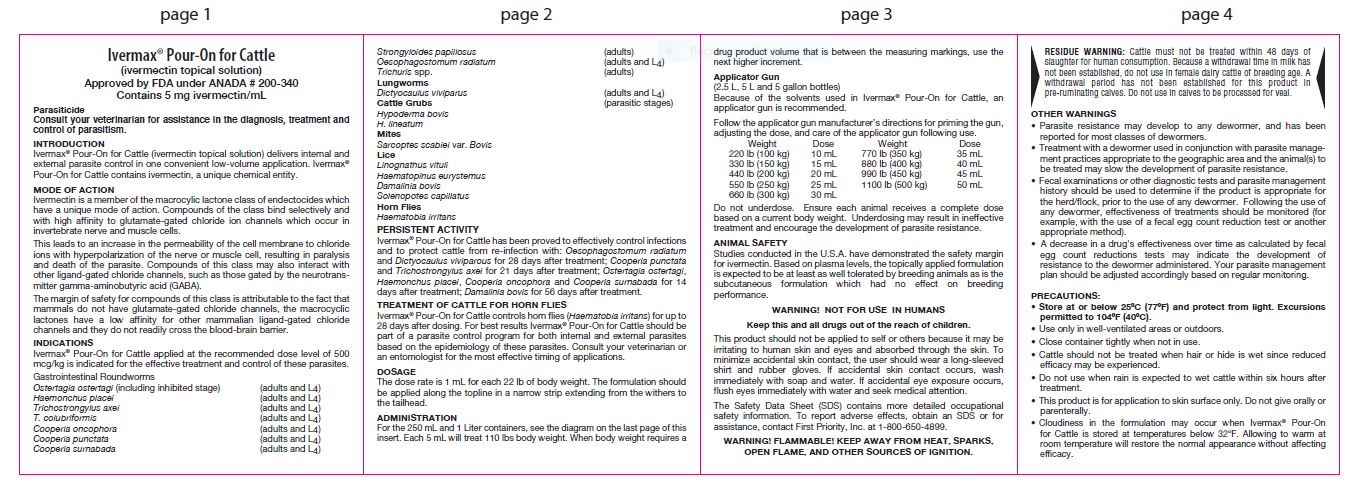
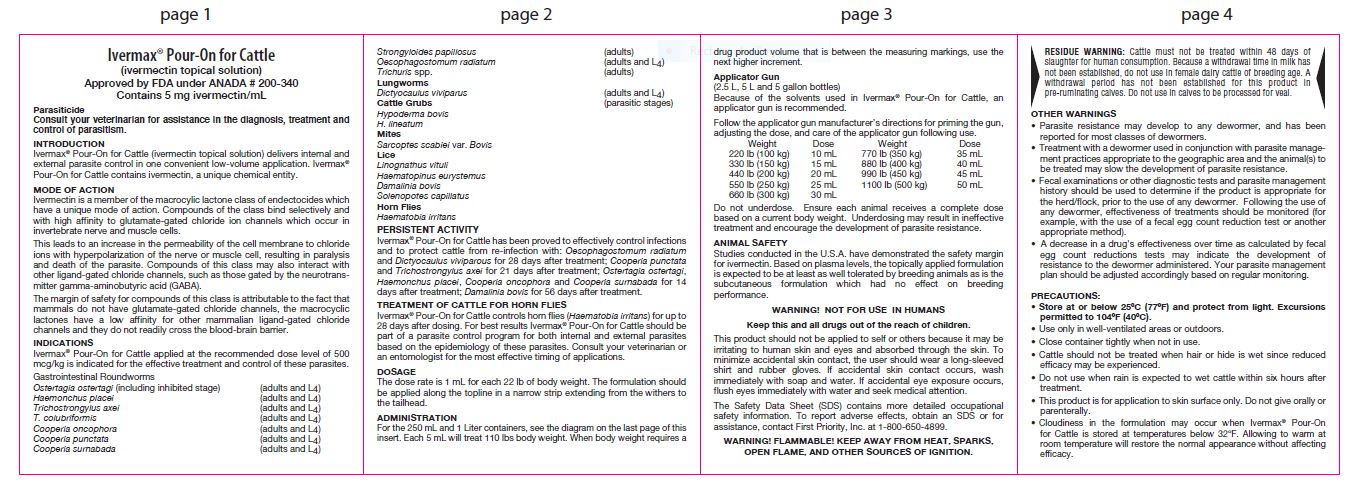
Ivermectin is a member of the macrocylic lactone class of endectocides which have a unique mode of action. Compounds of the class bind selectively and with high affinity to glutamate-gated chloride ion channels which occur in invertebrate nerve and muscle cells.
This leads to an increase in the permeability of the cell membrane to chloride ions with hyperpolarization of the nerve or muscle cell, resulting in paralysis and death of the parasite. Compounds of this class may also interact with other ligand-gated chloride channels, such as those gated by the neurotransmitter gamma-aminobutyric acid (GABA).
The margin of safety for compounds of this class is attributable to the fact that mammals do not have glutamate-gated chloride channels, the macrocyclic lactones have a low affinity for other mammalian ligand-gated chloride channels and they do not readily cross the blood-brain barrier.
-
Indications
Ivermax® Pour-On for Cattle applied at the recommended dose level of 500 mcg/kg is indicated for the effective treatment and control of these parasites.
Gastrointestinal Roundworms
Ostertagia ostertagi (including inhibited stage) (adults and L4)
Haemonchus placei (adults and L4)
Trichostrongylus axei (adults and L4)
T. colubriformis (adults and L4)
Cooperia oncophora (adults and L4)
Cooperia punctate (adults and L4)
Cooperia surnabada (adults and L4)
Strongyloides papillosu (adults)
Oesophagostomum radiatum (adults and L4)
Trichuris spp. (adults)Lungworms
Dictyocaulus viviparus (adults and L4)
Cattle Grubs (parasitic stages)Hypoderma bovis
H. lineatumMites
Sarcoptes scabiei var. Bovis
Lice
Linognathus vituli
Haematopinus eurystemus
Damalinia bovis
Solenopotes capillatusHorn Flies
Haematobia irritans
Persistent Activity
Ivermax® Pour-On for Cattle has been proved to effectively control infections and to protect cattle from re-infection with: Oesophagostomum radiatum and Dictyocaulus viviparous for 28 days after treatment; Cooperia punctata and Trichostrongylus axei for 21 days after treatment; Ostertagia ostertagi, Haemonchus placei, Cooperia oncophora and Cooperia surnabada for 14 days after treatment; Damalinia bovis for 56 days after treatment.
TREATMENT OF CATTLE FOR HORN FLIES
Ivermax® Pour-On for Cattle controls horn flies (Haematobia irritans) for up to 28 days after dosing. For best results Ivermax® Pour-On for Cattle should be part of a parasite control program for both internal and external parasites based on the epidemiology of these parasites. Consult your veterinarian or an entomologist for the most effective timing of applications. -
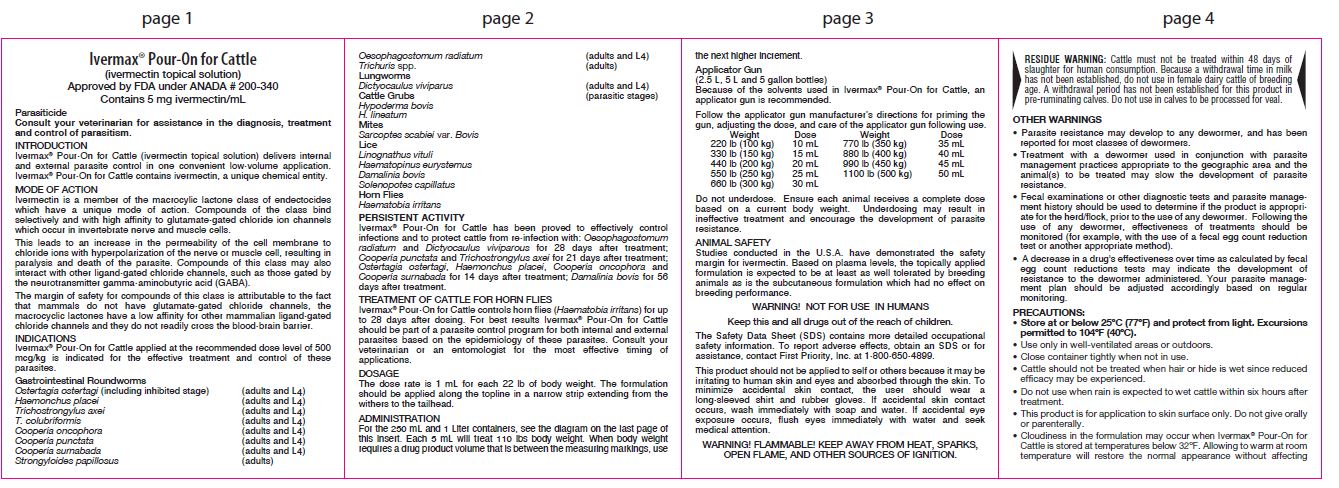
DOSAGE
The dose rate is 1 mL for each 22 lb of body weight. The formulation should be applied along the topline in a narrow strip extending from the withers to the tailhead.
ADMINISTRATION
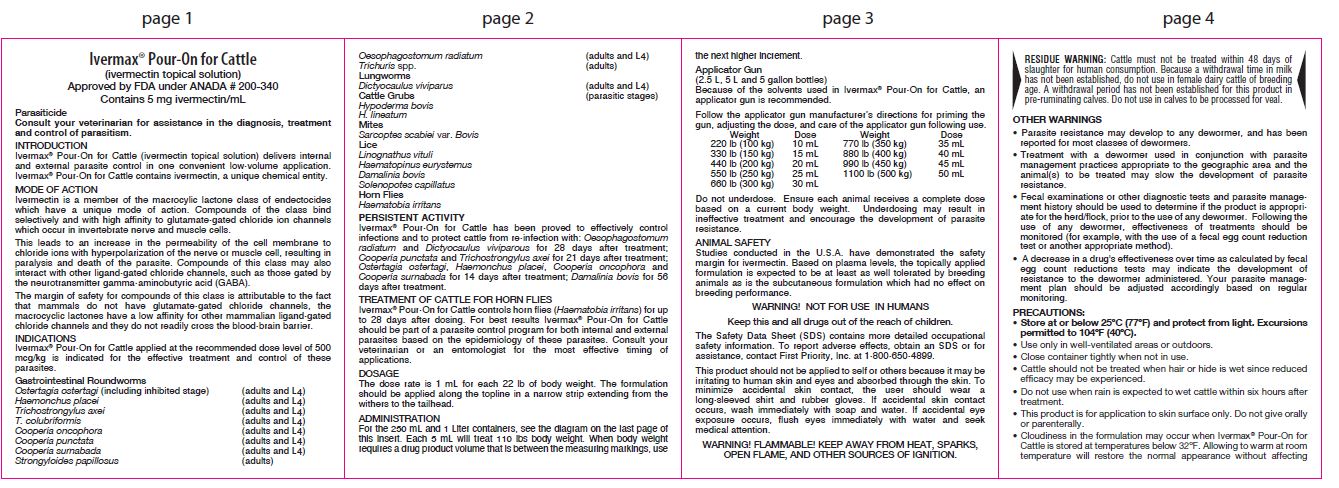
For the 250 mL and 1 Liter containers, see the diagram on the last page of this insert. Each 5 mL will treat 110 lbs body weight. When body weight requires a drug product volume that is between the measuring markings, use the next higher increment.
Applicator Gun
(2.5 L, 5 L and 5 gallon bottles)Because of the solvents used in Ivermax® Pour-On for Cattle, an applicator gun is recommended.
Follow the applicator gun manufacturer’s directions for priming the gun, adjusting the dose, and care of the applicator gun following use.Weight Dose 220 lb (100 kg) 10 mL 330 lb (150 kg) 15 mL 440 lb (200 kg) 20 mL 550 lb (250 kg) 25 mL 660 lb (300 kg) 30 mL 770 lb (350 kg) 35 mL 880 lb (400 kg) 40 mL 990 lb (450 kg) 45 mL 1100 lb (500 kg) 50 mL Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment and encourage the development of parasite resistance.
-
ANIMAL SAFETY
Studies conducted in the U.S.A. have demonstrated the safety margin for ivermectin. Based on plasma levels, the topically applied formulation is expected to be at least as well tolerated by breeding animals as is the subcutaneous formulation which had no effect on breeding performance.
WARNING! NOT FOR USE IN HUMANS
Keep this and all drugs out of the reach of children.
The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse effects, obtain an SDS or for assistance, contact First Priority, Inc. at 1-800-650-4899.
WARNING! FLAMMABLE! KEEP AWAY FROM
HEAT, SPARKS, OPEN FLAME, AND OTHER
SOURCES OF IGNITION.
This product should not be applied to self or others because it may be irritating to human skin and eyes and absorbed through the skin. To minimize accidental skin contact, the user should wear a long-sleeved shirt and rubber gloves. If accidental skin contact occurs, wash immediately with soap and water. If accidental eye exposure occurs, flush eyes immediately with water and seek medical attention.
- RESIDUE WARNING
-
OTHER WARNINGS
• Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
• Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
• Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatments should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
• A decrease in a drug's effectiveness over time as calculated by fecal egg count reductions tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring. -
PRECAUTIONS:
• Store at or below 25ºC (77ºF) and protect from light. Excursions permitted to 104ºF (40ºC).
• Use only in well-ventilated areas or outdoors.
• Close container tightly when not in use.
• Cattle should not be treated when hair or hide is wet since reduced efficacy may be experienced.
• Do not use when rain is expected to wet cattle within six hours after treatment.
• This product is for application to skin surface only. Do not give orally or parenterally.
• Cloudiness in the formulation may occur when Ivermax® Pour-On for Cattle is stored at temperatures below 32ºF. Allowing to warm at room temperature will restore the normal appearance without affecting efficacy.
• Antiparasitic activity of ivermectin will be impaired if the formulation is applied to areas of the skin with mange scabs or lesions, or with dermatosis or adherent materials, e.g., caked mud or manure.
• Ivermectin has been associated with adverse reactions in sensitive dogs; therefore, Ivermax® Pour-On for Cattle is not recommended for use in species other than cattle.
• Restricted Drug (California) – Use only as directed. -
When to Treat Cattle with Grubs
Ivermax® Pour-On for Cattle effectively controls all stages of cattle grubs. However, proper timing of treatment is important. For the most effective results, cattle should be treated as soon as possible after the end of the heel fly (warble fly) season. While this is not peculiar to ivermectin, destruction of Hypoderma larvae (cattle grubs) at the period when these grubs are in vital areas may cause undesirable host-parasite reactions. Killing Hypoderma lineatum when it is in the esophageal tissues may cause bloat; killing H. bovis when it is in the vertebral canal may cause staggering or paralysis. Cattle should be treated either before or after these stages of grub development.
Cattle treated with Ivermax® Pour-On for Cattle at the end of the fly season may be re-treated with Ivermax® Pour-On for Cattle during the winter without danger of grub-related reactions. For further information and advice on a planned parasite control program, consult your veterinarian. -
Environmental Safety
Studies indicate that when ivermectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive over time. Free ivermectin may adversely affect fish and certain aquatic organisms. Do not permit cattle to enter lakes, streams or ponds for at least six hours after treatment. Do not contaminate water by direct application or by the improper disposal of drug containers. Dispose of containers in an approved landfill or by incineration.
As with other avermectins, ivermectin is excreted in the dung of treated animals and can inhibit the reproduction and growth of pest and beneficial insects that use dung as a source of food and for reproduction. The magnitude and duration of such effects are species and life-cycle specific. When used according to label directions, the product is not expected to have an adverse impact on populations of dung-dependent insects. -
HOW SUPPLIED:
Ivermax® Pour-On for Cattle is available in 250 mL (8.5 fl oz) and 1 L (33.8 fl oz) tip and measure bottles, and 2.5 L (84.5 fl oz), 5 L (169.0 fl oz), and 5 Gallon (639.0 fl oz) containers for use with an appropriate automatic dosing applicator.


Manufactured for:
Aspen Veterinary Resources,® Ltd.
Liberty, MO 64068, USA A190FP 12/20
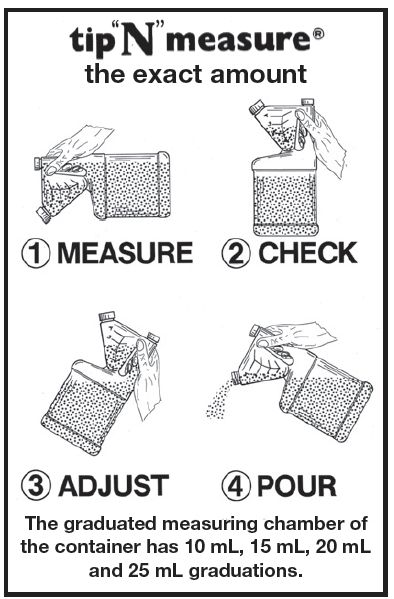
www.aspenveterinaryresources.com - DIAGRAM OF DEVICE
- 250 mL (8.5 fl oz)
- 1 Liter (33.8 fl oz)
- 2.5 Liter (rev. 12/20)
-
INGREDIENTS AND APPEARANCE
IVERMAX POUR-ON FOR CATTLE
ivermectin cattle pour-on solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:46066-302 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46066-302-02 12 in 1 CASE 1 250 mL in 1 BOTTLE, PLASTIC 2 NDC:46066-302-34 12 in 1 CASE 2 1000 mL in 1 BOTTLE, PLASTIC 3 NDC:46066-302-25 4 in 1 CASE 3 2500 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200340 01/30/2017 Labeler - ASPEN VETERINARY RESOURCES, LTD. (627265361) Establishment Name Address ID/FEI Business Operations First Priority Incorporated 179925722 manufacture, label