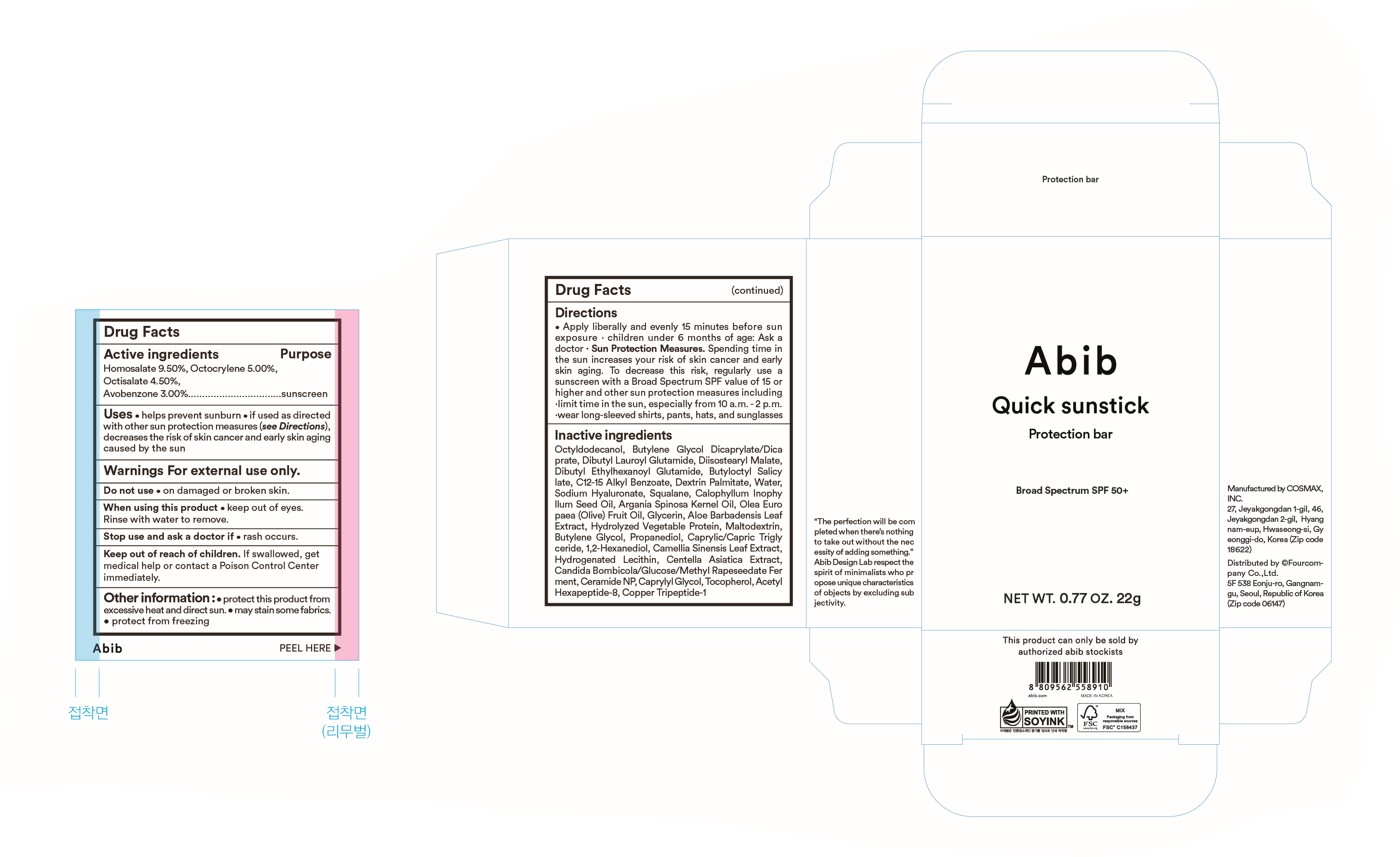
Label: ABIB QUICK SUNSTICK PROTECTION BAR- homosalate,octocrylene,octisalate,avobenzone stick
- NDC Code(s): 73676-216-20, 73676-216-40
- Packager: FOURCOMPANY CO., LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- SPL UNCLASSIFIED SECTION
-
DOSAGE & ADMINISTRATION
Directions apply liberally and evenly 15 minutes before sun exposure. reapply: after 80 minutes of swimming or sweating. immediately after towel drying. at least every 2 hours. children under 6 months of age: ask a doctor. Sun protection measures. spending time in the sun increases your risk of skin cancer and early aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m., wear long-sleeved shirts, pants, hats, and sunglasses
-
INACTIVE INGREDIENT
Actives ingredients Octyldodecanol, Butylene Glycol Dicaprylate/Dicaprate, Dibutyl Lauroyl Glutamide, Diisostearyl Malate, Dibutyl Ethylhexanoyl Glutamide, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Dextrin Palmitate, Water, Sodium Hyaluronate, Squalane, Calophyllum Inophyllum Seed Oil, Argania Spinosa Kegnel Oil, Olea Euro paea (Olive) Fruit Oil, Glycerin, Aloe Barbadensis Leaf Extract, Hydrolyzed Vegetable Protein, Maltodextrin, Butylene Glycol, Propanediol, Caprylic/Capric Trigly ceride, 1,2-Hexanediol, Camellia Sinensis Leaf Extract, Hydrogenated Lecithin, Centella Asiatica Extract, Candida Bombicola/Glucose/Methyl Rapeseedate Fer ment, Ceramide NP, Caprylyl Glycol, Tocopherol, Acetyl Hexapeptide-8, Copper Tripeptide-1
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ABIB QUICK SUNSTICK PROTECTION BAR
homosalate,octocrylene,octisalate,avobenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73676-216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9.5 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OLIVE OIL (UNII: 6UYK2W1W1E) HYALURONATE SODIUM (UNII: YSE9PPT4TH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) GLYCERIN (UNII: PDC6A3C0OX) TOCOPHEROL (UNII: R0ZB2556P8) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) SQUALANE (UNII: GW89575KF9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) ARGAN OIL (UNII: 4V59G5UW9X) GREEN TEA LEAF (UNII: W2ZU1RY8B0) OCTYLDODECANOL (UNII: 461N1O614Y) MALTODEXTRIN (UNII: 7CVR7L4A2D) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPANEDIOL (UNII: 5965N8W85T) PREZATIDE COPPER (UNII: 6BJQ43T1I9) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) TAMANU OIL (UNII: JT3LVK84A1) CERAMIDE NP (UNII: 4370DF050B) ALOE VERA LEAF (UNII: ZY81Z83H0X) CENTELLA ASIATICA (UNII: 7M867G6T1U) HYDROLYZED SENEGALIA MACROSTACHYA SEED PROTEIN (ENZYMATIC; 800 MW) (UNII: 49W7E9YC8L) STARMARELLA BOMBICOLA (UNII: V4PN2TU66C) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) METHYL RAPESEEDATE (UNII: QA2Q2EXS63) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73676-216-40 1 in 1 BOX 06/05/2024 1 NDC:73676-216-20 22 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/05/2024 Labeler - FOURCOMPANY CO., LTD. (694864584) Establishment Name Address ID/FEI Business Operations Cosmax, Inc. 689049693 manufacture(73676-216)