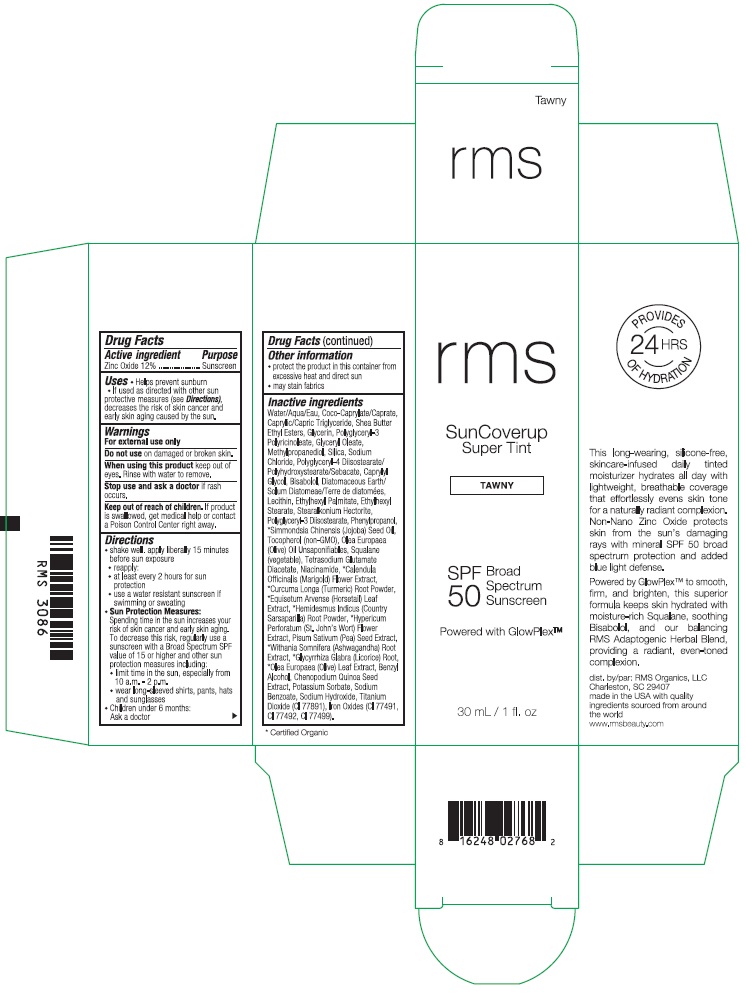
Label: RMS SUNCOVERUP SUPER TINT SPF 50 TAWNY- zinc oxide lotion
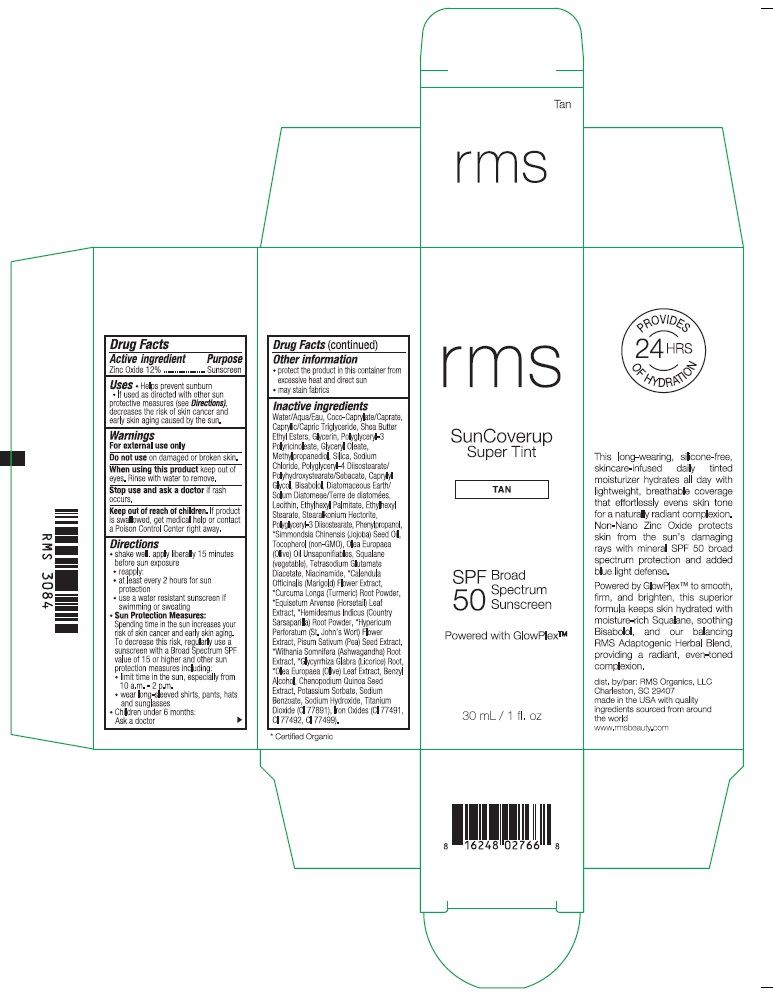
RMS SUNCOVERUP SUPER TINT SPF 50 TAN- zinc oxide lotion
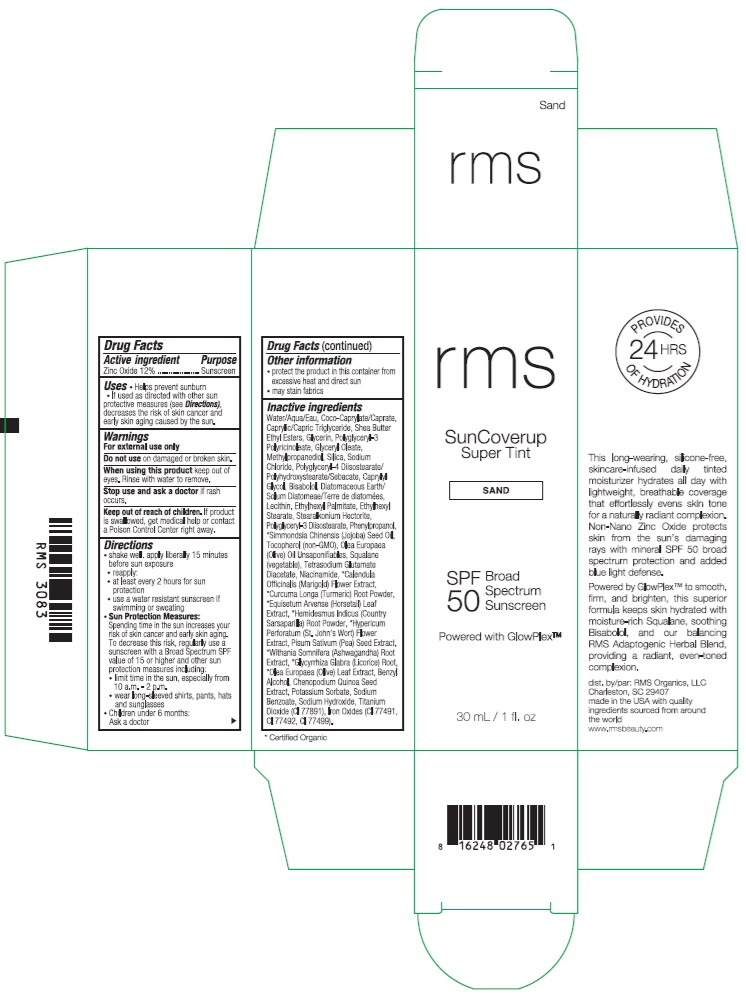
RMS SUNCOVERUP SUPER TINT SPF 50 SAND- zinc oxide lotion
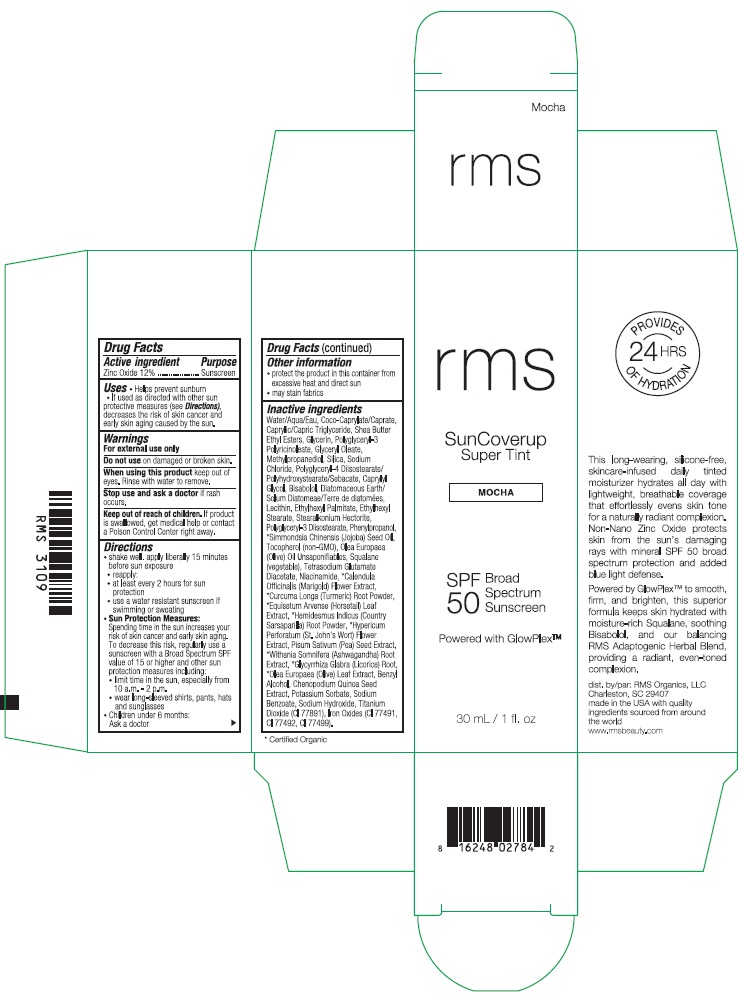
RMS SUNCOVERUP SUPER TINT SPF 50 MOCHA- zinc oxide lotion
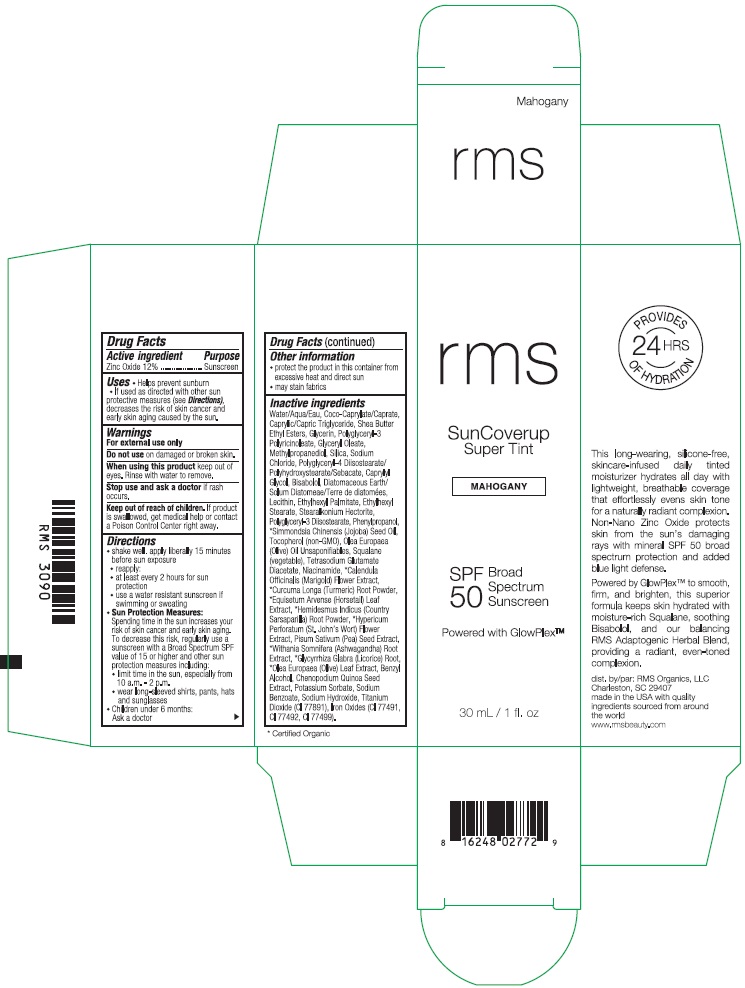
RMS SUNCOVERUP SUPER TINT SPF 50 MAHOGANY- zinc oxide lotion
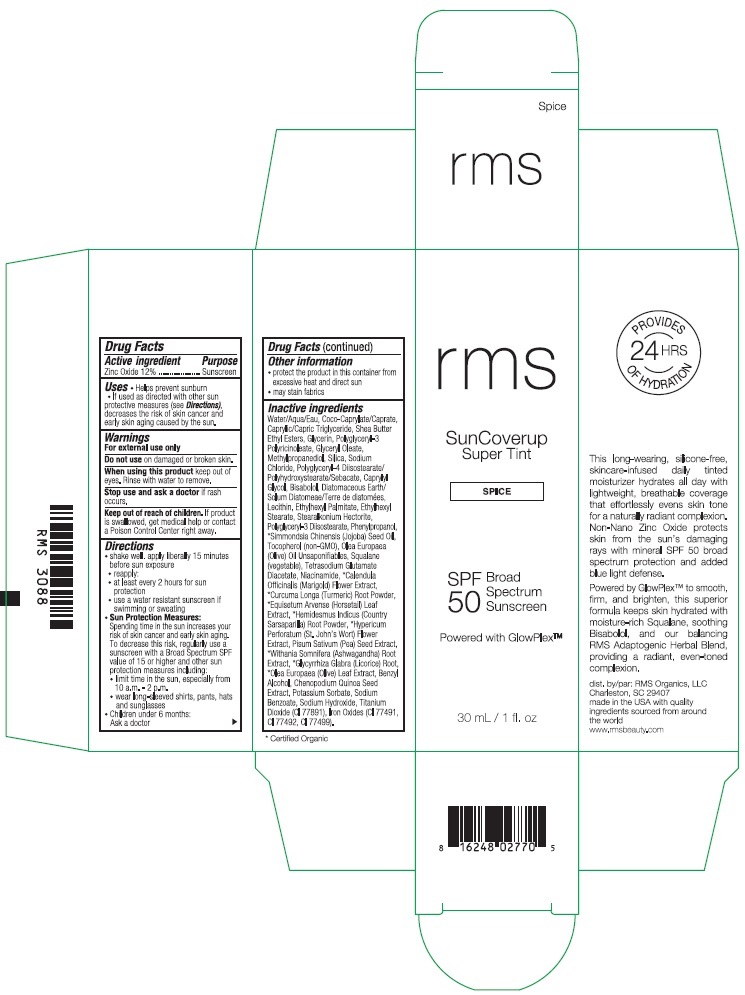
RMS SUNCOVERUP SUPER TINT SPF 50 SPICE- zinc oxide lotion
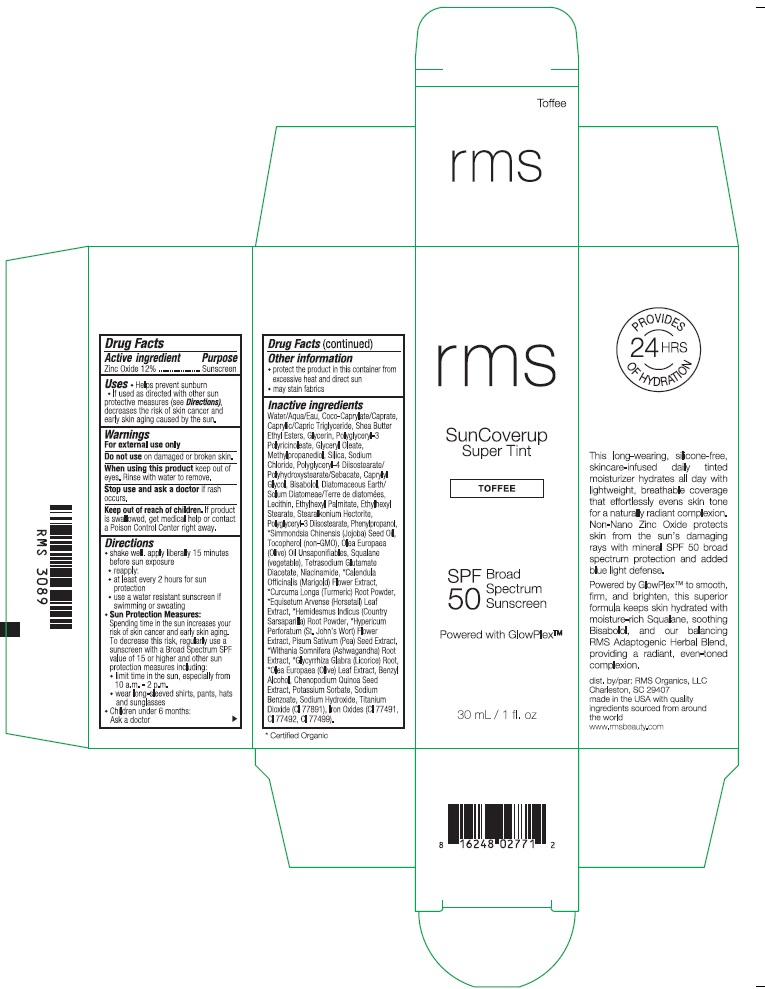
RMS SUNCOVERUP SUPER TINT SPF 50 TOFFEE- zinc oxide lotion
-
NDC Code(s):
83249-010-01,
83249-011-01,
83249-012-01,
83249-013-01, view more83249-014-01, 83249-015-01, 83249-016-01
- Packager: Rms organics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- shake well. apply liberally 15 minutes before sun exposure
- reapply: at least every 2 hours for sun protection
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Water/Aqua/Eau, Coco-Caprylate/Caprate, Caprylic/Capric Triglyceride, Shea Butter Ethyl Esters, Glycerin, Polyglyceryl-3 Polyricinoleate, Glyceryl Oleate, Methylpropanediol, Silica, Sodium Chloride, Polyglyceryl-4 Diisostearate/Polyhydroxystearate/Sebacate, Caprylyl Glycol, Bisabolol, Diatomaceous Earth/Solum Diatomeae/Terra de diatomees, Lecithin, Ethylhexyl Palmitate, Ethylhexyl Stearate, Stearalkonium Hectorite, Polyglyceryl-3 Diisostearate, Phenylpropanol, *Simmondsia Chinensis (Jojoba) Seed Oil, Tocopherol (non-GMO), Olea Europaea (Olive) Oil Unsaponifiables, Squalane (vegetable), Tetrasodium Glutamate Diacetate, Niacinamide, *Calendula Officinalis (Marigold) Flower Extract, *Curcuma Longa (Turmeric) Root Powder, *Equisetum Arvense (Horsetail) Leaf Extract, *Hemidesmus Indicus (Country Sarsaparilla) Root Powder, *Hypericum Perforatum (St. John's Wort) Flower Extract, Pisum Sativum (Pea) Seed Extract, *Withania Somnifera (Ashwagandha) Root Extract, *Glycyrrhiza Glabra (Licorice) Root, *Olea Europaea (Olive) Leaf Extract, Benzyl Alcohol, Chenopodium Quinoa Seed Extract, Potassium Sorbate, Sodium Benzoate, Sodium Hydroxide, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499).
*Certified Organic
- Company Information
- Product Packaging - Mahogany
- Product Packaging - Mocha
- Product Packaging - Sand
- Product Packaging - Spice
- Product Packaging - Tan
- Product Packaging - Tawny
- Product Packaging - Toffee
-
INGREDIENTS AND APPEARANCE
RMS SUNCOVERUP SUPER TINT SPF 50 TAWNY
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-015-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 TAN
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-014-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 SAND
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-012-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 MOCHA
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-011-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 MAHOGANY
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) JOJOBA OIL (UNII: 724GKU717M) NIACINAMIDE (UNII: 25X51I8RD4) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM HYDROXIDE (UNII: 55X04QC32I) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) GLYCERIN (UNII: PDC6A3C0OX) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-010-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 SPICE
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-013-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 RMS SUNCOVERUP SUPER TINT SPF 50 TOFFEE
zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83249-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 120 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-4 DIISOSTEARATE/POLYHYDROXYSTEARATE/SEBACATE (UNII: 687U3PEB2Y) LECITHIN, SUNFLOWER (UNII: 834K0WOS5G) SHEA BUTTER ETHYL ESTERS (UNII: V2CI786FPG) ETHYLHEXYL PALMITATE (UNII: 2865993309) SQUALANE (UNII: GW89575KF9) OLEA EUROPAEA (OLIVE) OIL UNSAPONIFIABLES (UNII: XO45V955LT) FERRIC OXIDE RED (UNII: 1K09F3G675) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) METHYLPROPANEDIOL (UNII: N8F53B3R4R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) TURMERIC (UNII: 856YO1Z64F) SODIUM CHLORIDE (UNII: 451W47IQ8X) LEVOMENOL (UNII: 24WE03BX2T) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TOCOPHEROL (UNII: R0ZB2556P8) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) FERROSOFERRIC OXIDE (UNII: XM0M87F357) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) EQUISETUM ARVENSE BRANCH (UNII: 1L0VKZ185E) HEMIDESMUS INDICUS ROOT (UNII: Y5CFT48S90) WITHANIA SOMNIFERA ROOT (UNII: V038D626IF) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) JOJOBA OIL (UNII: 724GKU717M) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENYLPROPANOL (UNII: 0F897O3O4M) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM HYDROXIDE (UNII: 55X04QC32I) BENZYL ALCOHOL (UNII: LKG8494WBH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PEA (UNII: W4X7H8GYFM) CHENOPODIUM QUINOA SEED (UNII: OO4K72NZ2X) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83249-016-01 1 in 1 CARTON 09/01/2024 1 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/01/2024 Labeler - Rms organics LLC (031351949)