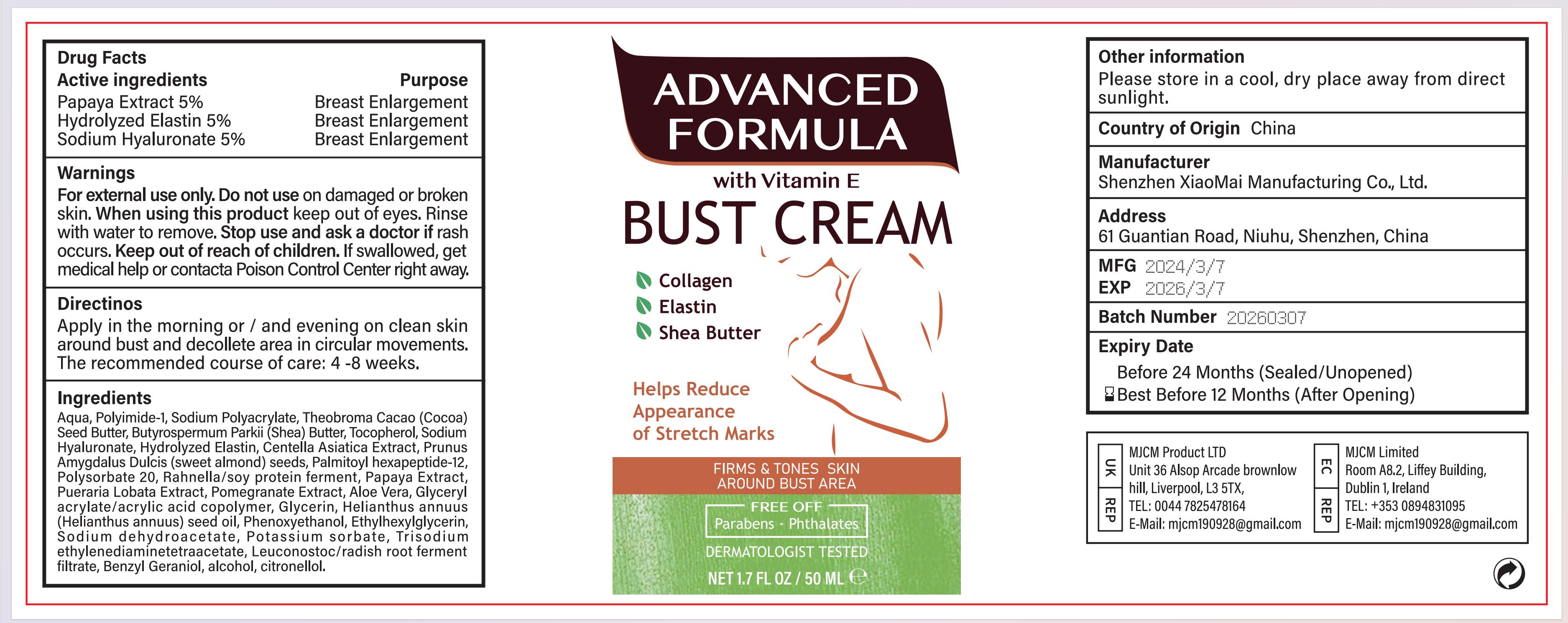
Label: VITAMIN E BUST CREAM cream
- NDC Code(s): 83872-171-01
- Packager: Shenzhen XiaoMai Manufacturing Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children.
- Directions for use
-
INACTIVE INGREDIENT
Aqua: 70%
Polyimide-1: 2%
Sodium Polyacrylate: 1%
Theobroma Cacao (Cocoa) Seed Butter: 3%
Butyrospermum Parkii (Shea) Butter: 3%
Tocopherol: 1%
Sodium Hyaluronate: 2%
Hydrolyzed Elastin: 2%
Centella Asiatica Extract: 2%
Prunus Amygdalus Dulcis (Sweet Almond) Seed: 2%
Palmitoyl Hexapeptide-12: 1%
Polysorbate 20: 1%
Rahnella/Soy Protein Ferment: 1%
Papaya Extract: 1%
Pueraria Lobata Extract: 1%
Pomegranate Extract: 1%
Aloe Vera: 1%
Glyceryl Acrylate/Acrylic Acid Copolymer: 2%
Glycerin: 2%
Helianthus Annuus (Sunflower) Seed Oil: 2%
Phenoxyethanol: 1%
Ethylhexylglycerin: 1%
Sodium Dehydroacetate: 1%
Potassium Sorbate: 1%
Trisodium Ethylenediaminetetraacetate: 1%
Leuconostoc/Radish Root Ferment Filtrate: 1%
Benzyl Geraniol: 0.5%
Alcohol: 1%
Citronellol: 0.5% - Other Information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN E BUST CREAM
vitamin e bust cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83872-171 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PAPAYA (UNII: KU94FIY6JB) (PAPAYA - UNII:KU94FIY6JB) PAPAYA 50 mg in 1 g Inactive Ingredients Ingredient Name Strength COCOA (UNII: D9108TZ9KG) 30 mg in 1 g PUERARIA TUBEROSA ROOT (UNII: 3UW5OG233A) 10 mg in 1 g AQUA REGIA (UNII: X3TT5X989E) 700 mg in 1 g GLYCERYL ACRYLATE/ACRYLIC ACID COPOLYMER (300000 CP AT 2%) (UNII: MEA9KH24QG) 20 mg in 1 g CENTELLA ASIATICA (UNII: 7M867G6T1U) 20 mg in 1 g SUNFLOWER OIL (UNII: 3W1JG795YI) 20 mg in 1 g SODIUM POLYACRYLATE AMYLOPECTIN (7 MICROMETER PARTICLE) (UNII: 8B966WW4PP) 10 mg in 1 g HYDROLYZED JOJOBA ESTERS (POTASSIUM SALTS) (UNII: CH428W5O62) 20 mg in 1 g POMEGRANATE (UNII: 56687D1Z4D) 10 mg in 1 g PHENOXYETHANOL (UNII: HIE492ZZ3T) 10 mg in 1 g ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 10 mg in 1 g SODIUM DEHYDROACETATE (UNII: 8W46YN971G) 10 mg in 1 g POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 10 mg in 1 g SHEANUT OIL (UNII: O88E196QRF) 30 mg in 1 g .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) 10 mg in 1 g PRUNUS APETALA FRUIT (UNII: PY2KV3CJJL) 20 mg in 1 g BUTTER SOLE (UNII: 12M4IUY1BF) 30 mg in 1 g POLYSORBATE 20 (UNII: 7T1F30V5YH) 10 mg in 1 g GLYCERIN (UNII: PDC6A3C0OX) 20 mg in 1 g PEG-9 DIGLYCIDYL ETHER/SODIUM HYALURONATE CROSSPOLYMER (UNII: 788QAG3W8A) 20 mg in 1 g ALOE VERA LEAF (UNII: ZY81Z83H0X) 10 mg in 1 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83872-171-01 50 g in 1 BOTTLE; Type 0: Not a Combination Product 06/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/04/2024 Labeler - Shenzhen XiaoMai Manufacturing Co., Ltd. (712999147) Establishment Name Address ID/FEI Business Operations Shenzhen XiaoMai Manufacturing Co., Ltd. 712999147 manufacture(83872-171)