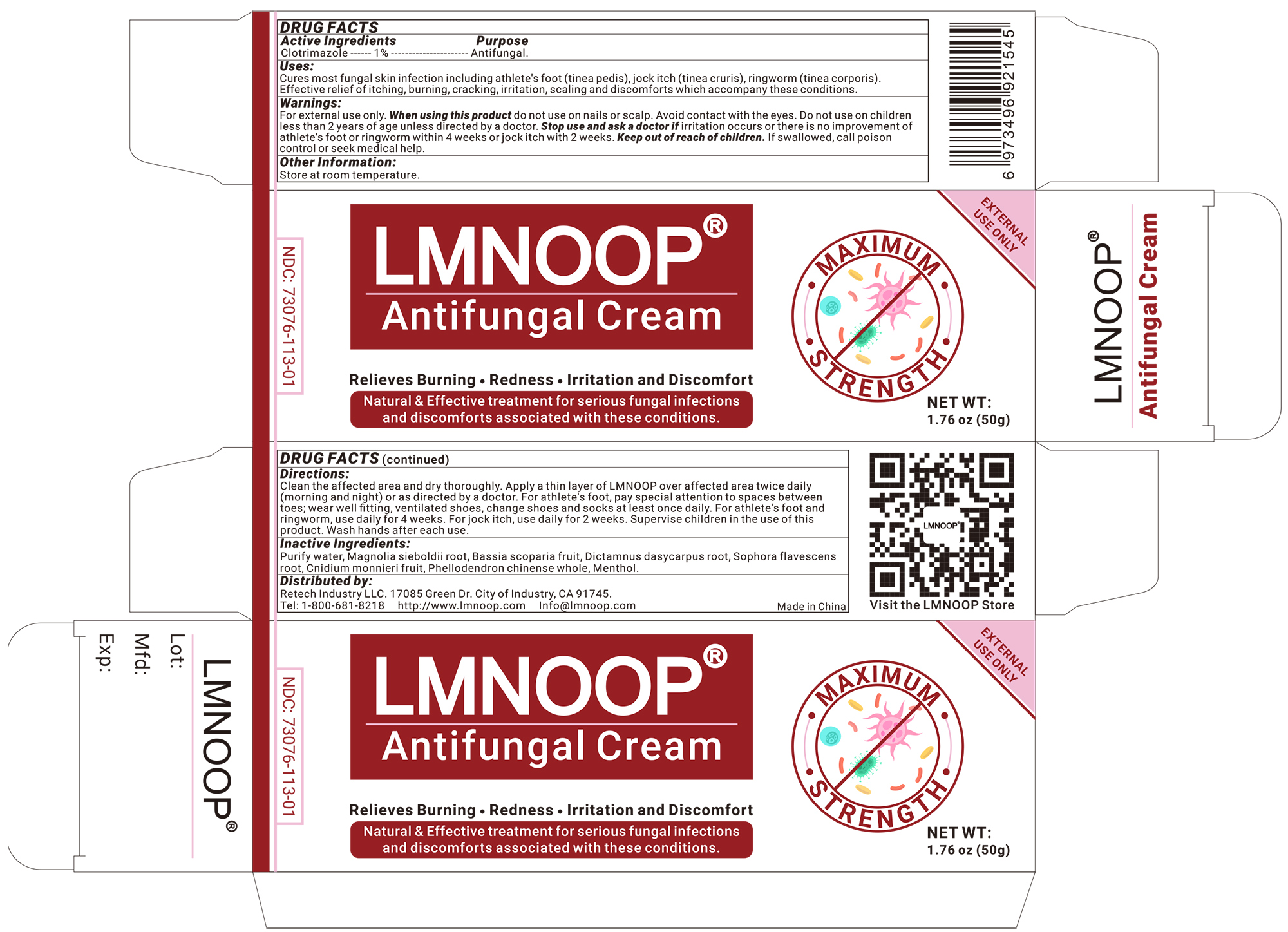
Label: LMNOOP ANTIFUNGAL CREAM cream
- NDC Code(s): 73076-147-01, 73076-147-02
- Packager: Shenzhen Ishan Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Inactive ingredients
- Keep out of reach of children.
- Consulting a doctor before using
- Stop use and ask a doctor if
- USES
- Other information
-
Directions
* Clean the affected area and dry thoroughly
* Apply a thin layer of LMNOOP over affected area twice daily (morning and night) or as directed by a doctor.
* Use daily for 4 weeks, pay special attention to the spaces between the toes; wear well-fitting ventilated shoes, and change shoes and socks at least once daily.
* For athlete's foot, pay special attention to spaces between toes; wear well fitting, ventilated shoes, change shoes and socks at least once daily.
* For athlete's foot and ringworm, use daily for 4 weeks.
* For jock itch, use daily for 2 weeks.
* Supervise children in the use of this product.
* Wash hands after each use. - Dosage & administration
- When using this product
- Warning
- Lable
-
INGREDIENTS AND APPEARANCE
LMNOOP ANTIFUNGAL CREAM
lmnoop antifungal cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73076-147 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength MAGNOLIA SIEBOLDII ROOT (UNII: F5O3IH7US3) 3 g in 100 g DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) 3 g in 100 g PHELLODENDRON CHINENSE WHOLE (UNII: QKA3ZK8IIE) 5 g in 100 g MENTHOL (UNII: L7T10EIP3A) 1 g in 100 g BASSIA SCOPARIA FRUIT (UNII: 04W97Z676Y) 3 g in 100 g SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) 5 g in 100 g CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) 3 g in 100 g WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73076-147-01 50 g in 1 TUBE; Type 0: Not a Combination Product 12/01/2022 2 NDC:73076-147-02 100 g in 1 BOTTLE; Type 0: Not a Combination Product 10/17/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/01/2022 Labeler - Shenzhen Ishan Technology Co., Ltd (554484192) Establishment Name Address ID/FEI Business Operations Shenzhen Ishan Technology Co., Ltd 554484192 manufacture(73076-147)