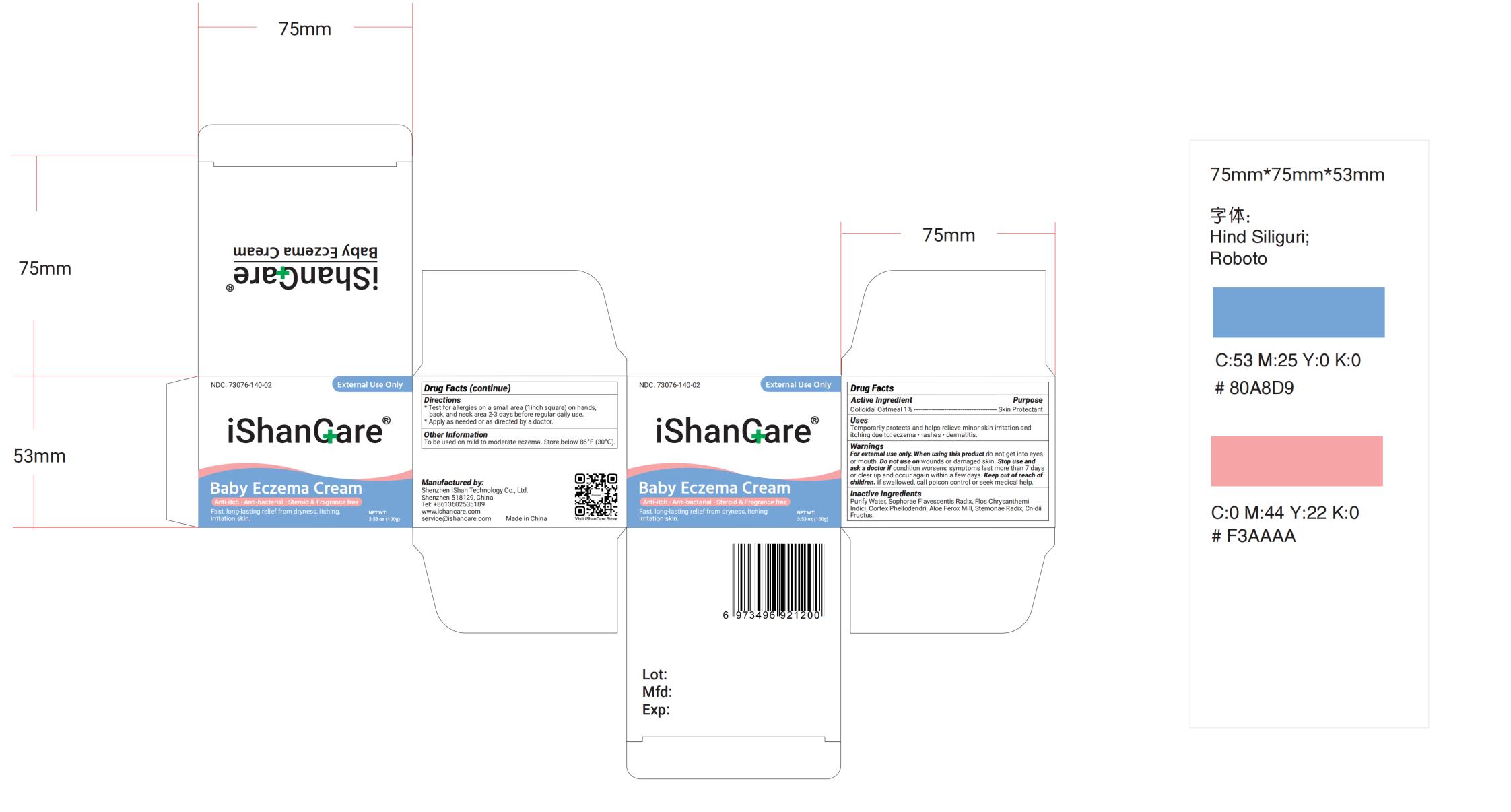
Label: LMNOOP BABY ECZEMA RELIEF CREAM- baby eczema relief cream cream
- NDC Code(s): 73076-141-02
- Packager: Shenzhen Ishan Technology Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Directions
- Active Ingredient
- Ask doctor
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- WHEN USING
-
WARNINGS
For external use only. When using this product
do not get into eyes or
mouth. Stop use and ask a doctor if condition worsens, symptoms last
more than 7 days or clear up and occur again within a few days.
Do not use
on deep puncture wounds, serious burns. Keep out of reach of children. If
swallowed, call poison control or seek medical help.
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- PURPOSE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LMNOOP BABY ECZEMA RELIEF CREAM
baby eczema relief cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73076-141 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 1 g in 100 g Inactive Ingredients Ingredient Name Strength CNIDIUM MONNIERI FRUIT (UNII: V1IA3S3CUS) 3 g in 100 g STEMONA JAPONICA ROOT (UNII: FXG254HF10) 0.5 g in 100 g PHELLODENDRON CHINENSIS BARK (UNII: 2866QMZ434) CHRYSANTHEMIN (UNII: 8X15R84UEM) 2 g in 100 g ALOE FEROX LEAF (UNII: 0D145J8EME) 0.5 g in 100 g SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) 1.5 g in 100 g WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73076-141-02 100 g in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 10/01/2023 Labeler - Shenzhen Ishan Technology Co., Ltd (554484192) Establishment Name Address ID/FEI Business Operations Shenzhen Ishan Technology Co., Ltd 554484192 manufacture(73076-141)