Label: SULFATRIMTM PEDIATRIC SUSPENSION- sulfamethoxazole and trimethoprim oral suspension liquid
- NDC Code(s): 80432-065-33
- Packager: TriRx Huntsville Pharmaceutical Services
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Description
- Inactive Ingredient Section
- Clinical Pharmacology
- Geriatric Pharmacokinetics
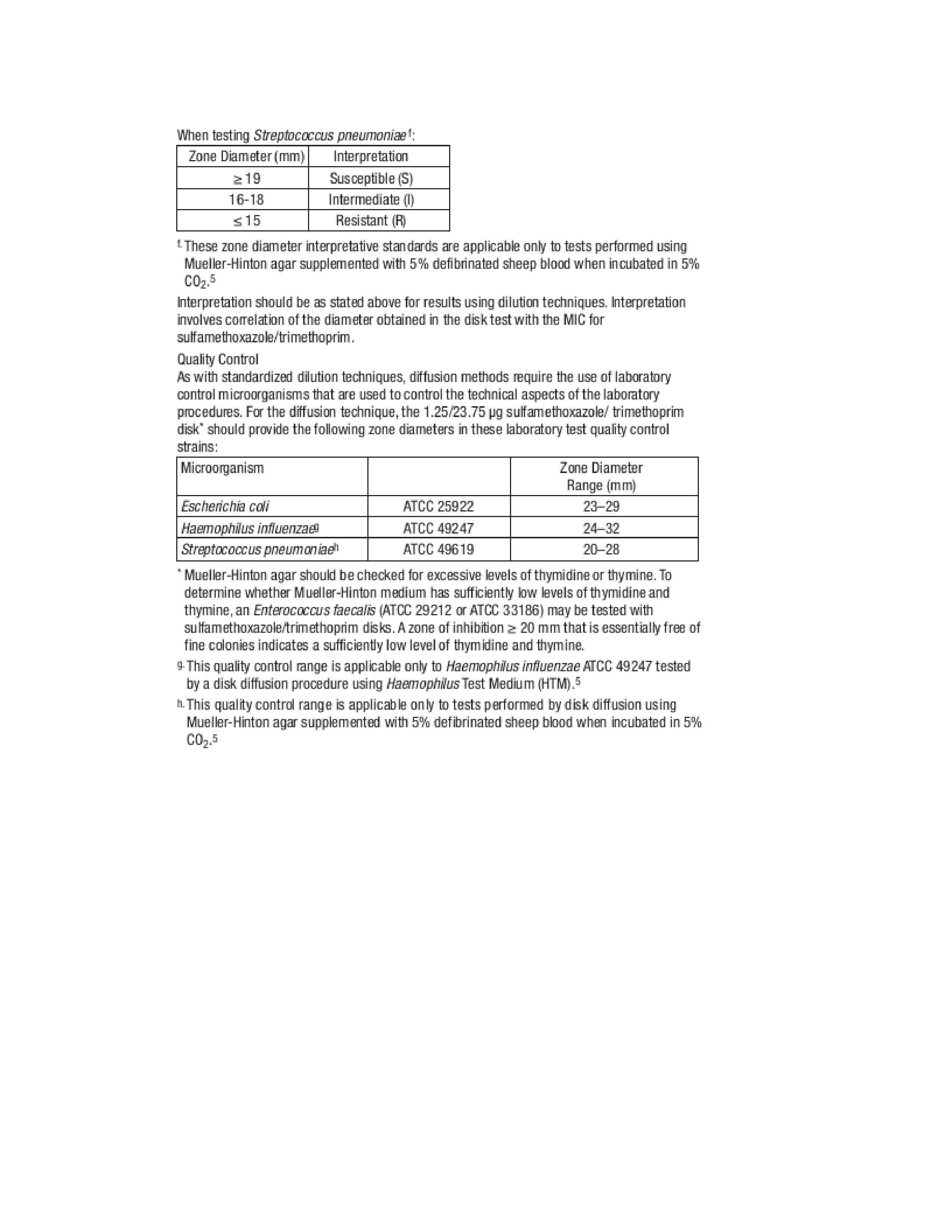
- Microbiology 2
- Microbiology 1
- Microbiology 3
- Indications and Usage
- Contraindications
- Warnings 1
- Warnings 2
- Precautions
- Information for Patients
- Laboratory Tests
- Drug Interactions
- Drug Laboratory Test Interactions
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy 1
- Pregnancy 2
- Geriatric Use
- Adverse Reactions 1
- Adverse Reactions 2
- Overdosage
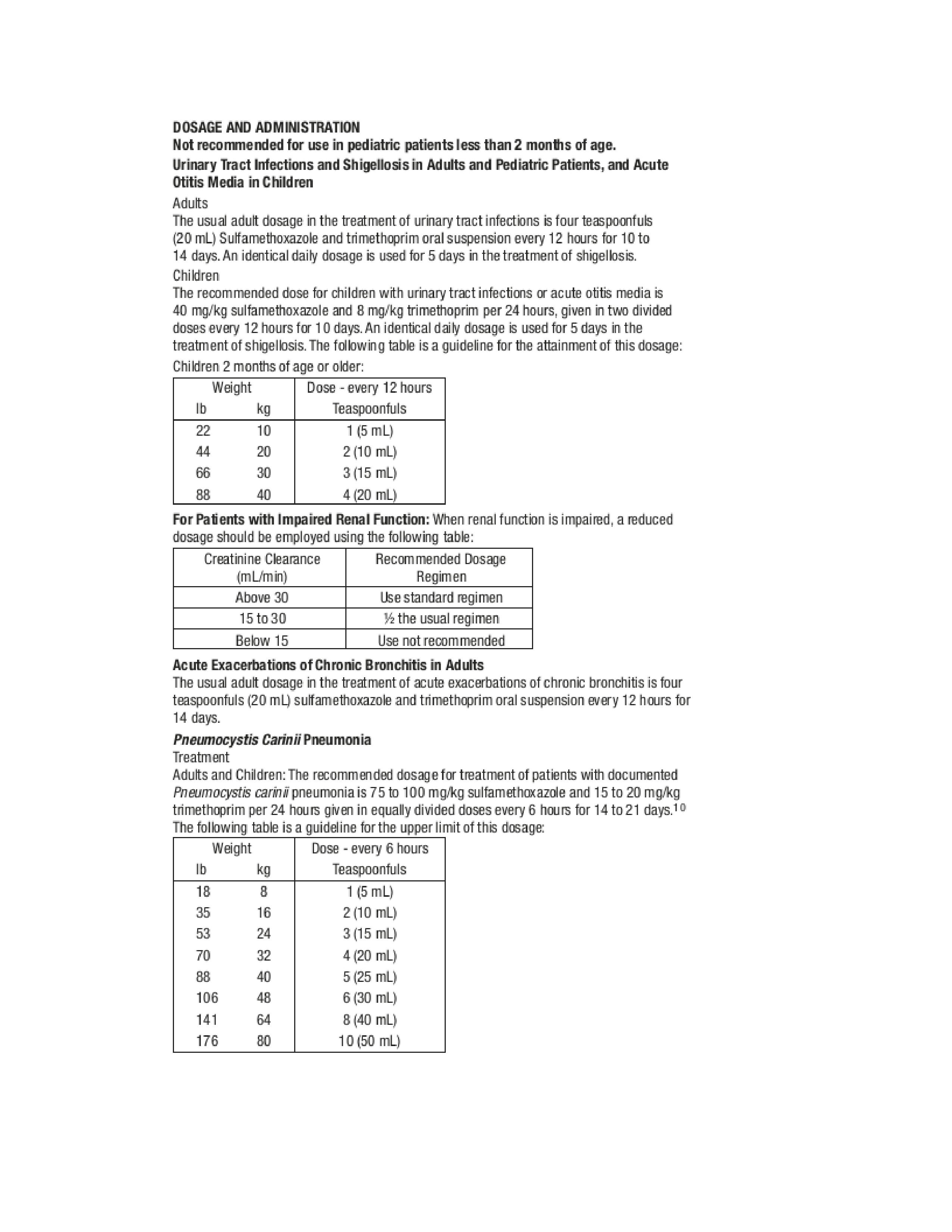
- Dosage and Administration 1
- Dosage and Administration 2
- How Supplied
- References
- Label
-
INGREDIENTS AND APPEARANCE
SULFATRIMTM PEDIATRIC SUSPENSION
sulfamethoxazole and trimethoprim oral suspension liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80432-065 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 40 mg in 5 mL SULFAMETHOXAZOLE (UNII: JE42381TNV) (SULFAMETHOXAZOLE - UNII:JE42381TNV) SULFAMETHOXAZOLE 200 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SUCROSE (UNII: C151H8M554) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C RED NO. 40 (UNII: WZB9127XOA) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) ALCOHOL (UNII: 3K9958V90M) CHERRY (UNII: BUC5I9595W) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80432-065-33 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018615 04/29/2024 Labeler - TriRx Huntsville Pharmaceutical Services (117090286)