Label: LIDOCAINE AND PRILOCAINE CREAM cream
- NDC Code(s): 80432-053-65, 80432-053-71
- Packager: TriRx Huntsville Pharmaceutical Services
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
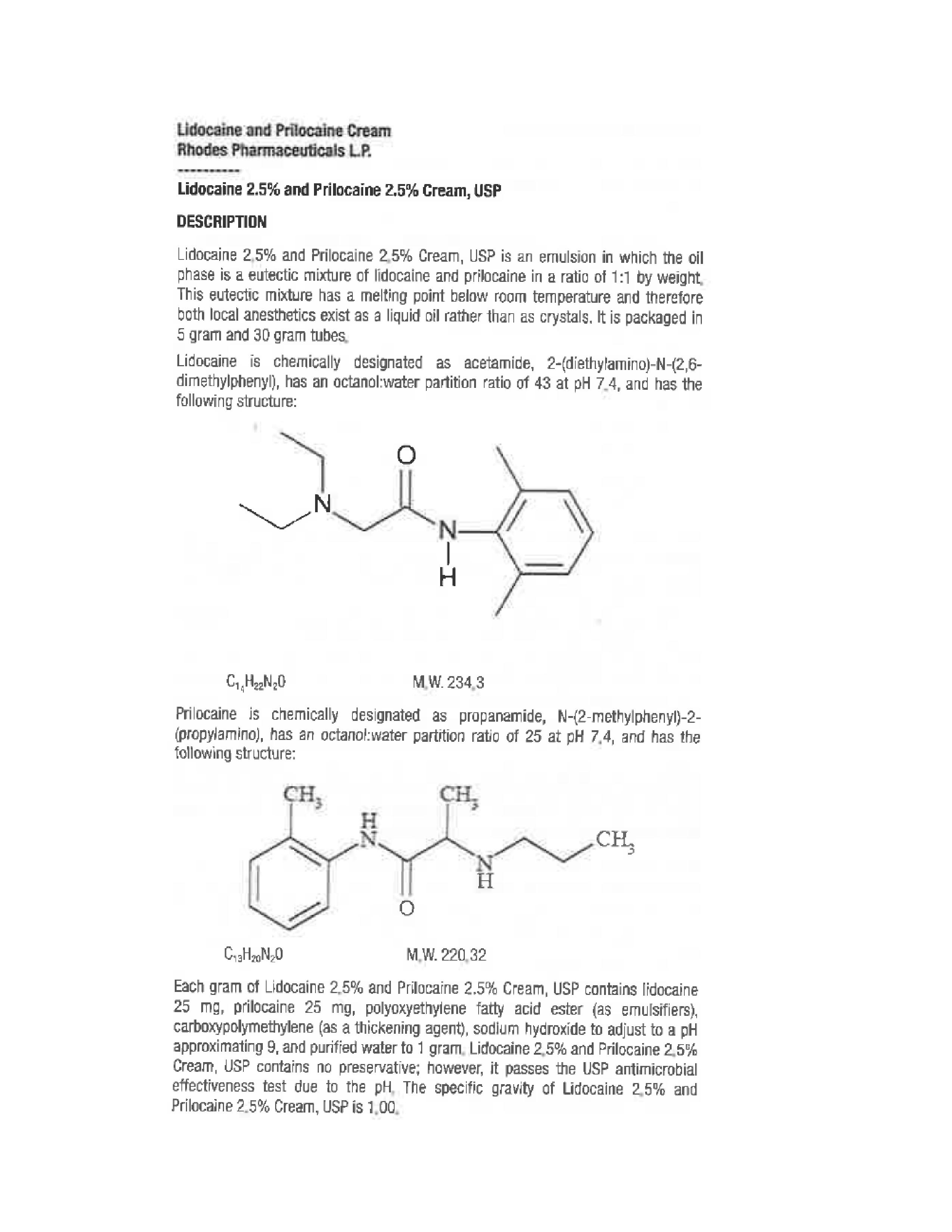
- Description
- Clinical Pharmacology
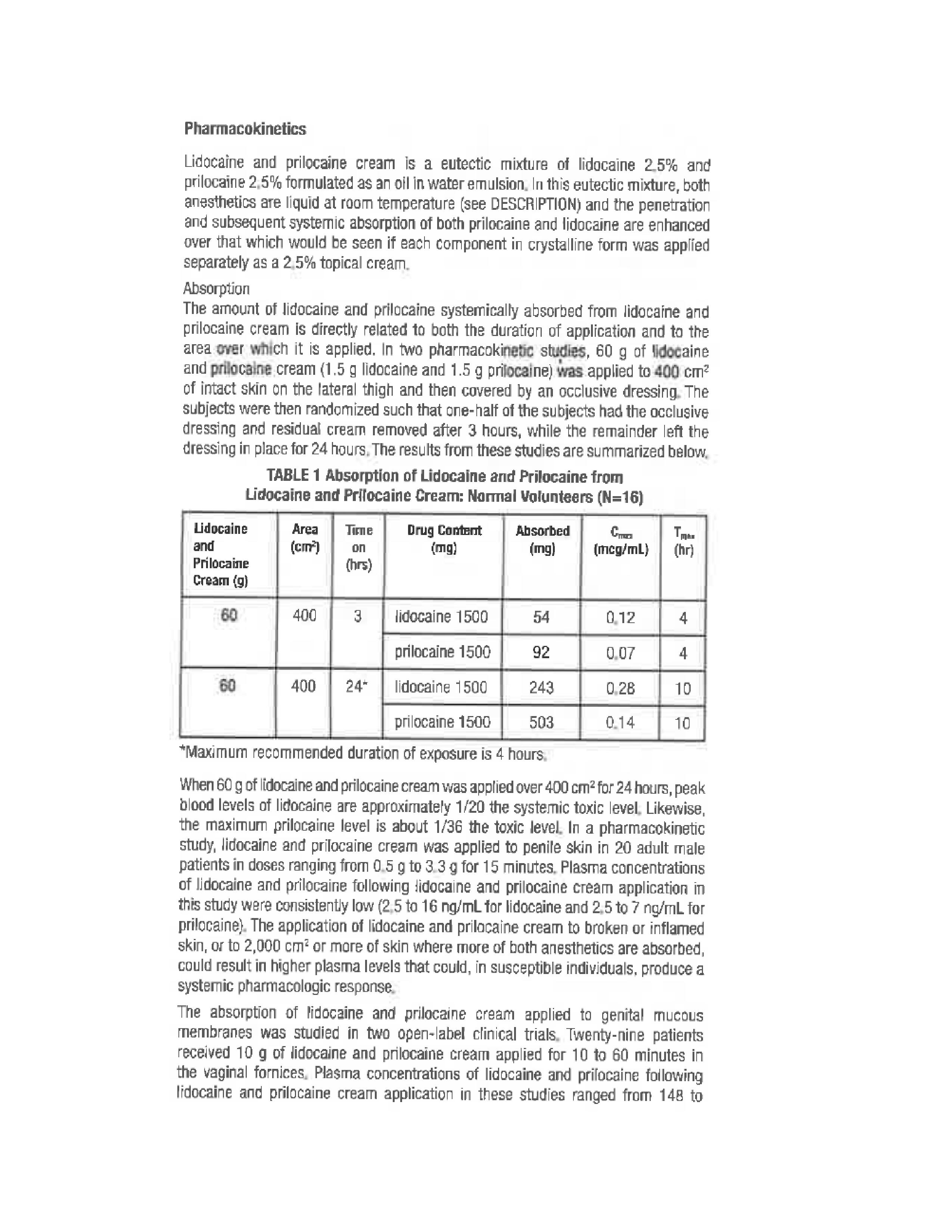
- Pharmacokinetics 1
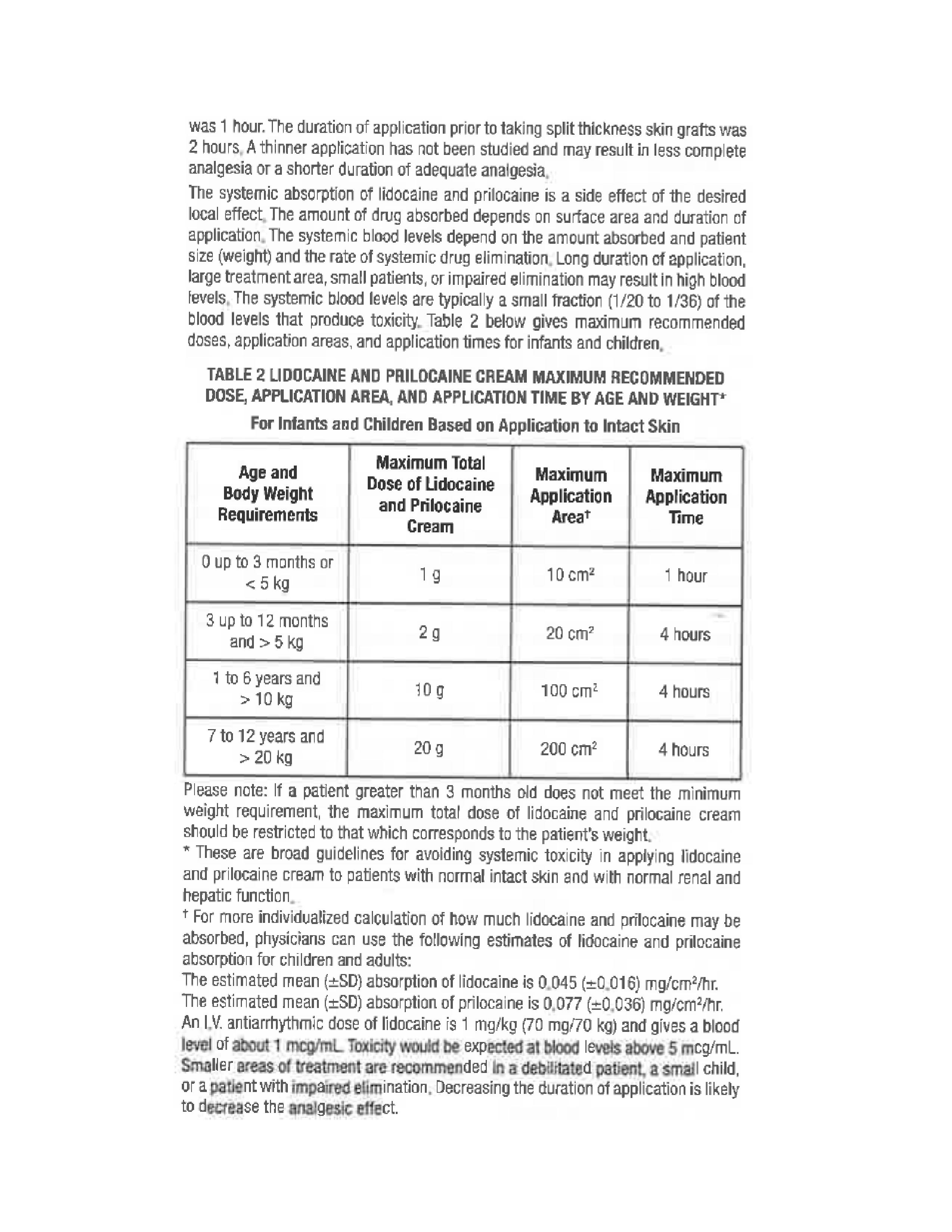
- Pharmacokinetics 2
- Clinical Studies
- Individualization of Dose 1
- Individualization of Dose 2
- Indications and Usage
- Contraindications
- Warnings
- Precautions
- Information for Patients
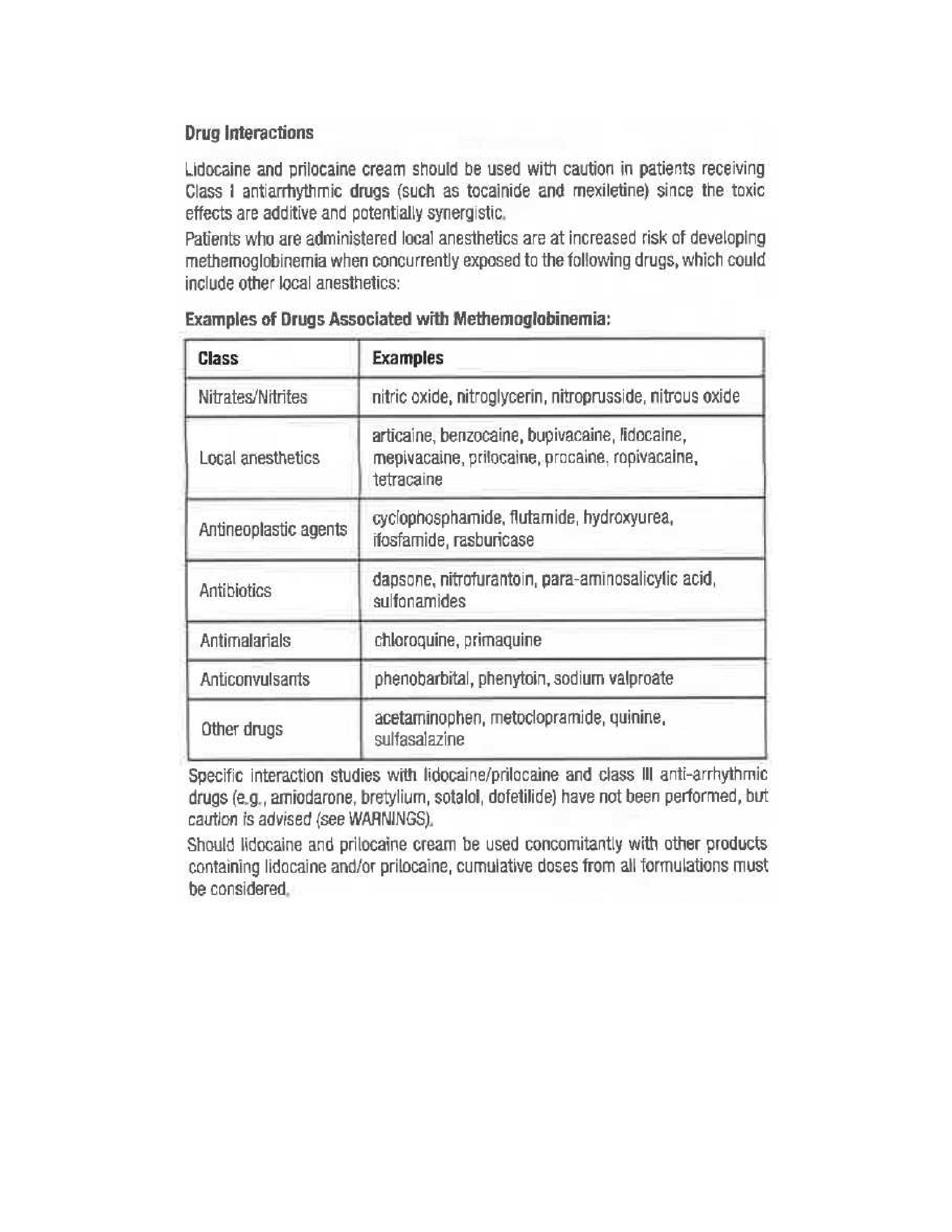
- Drug Interactions
- Use in Pregnancy
- Labor and Delivery
- Nursing Mothers
- Pediatric Use 1
- Pediatric Use 2
- Geriatric Use
- Adverse Reactions
- Overdosage
- Dosage and Administration
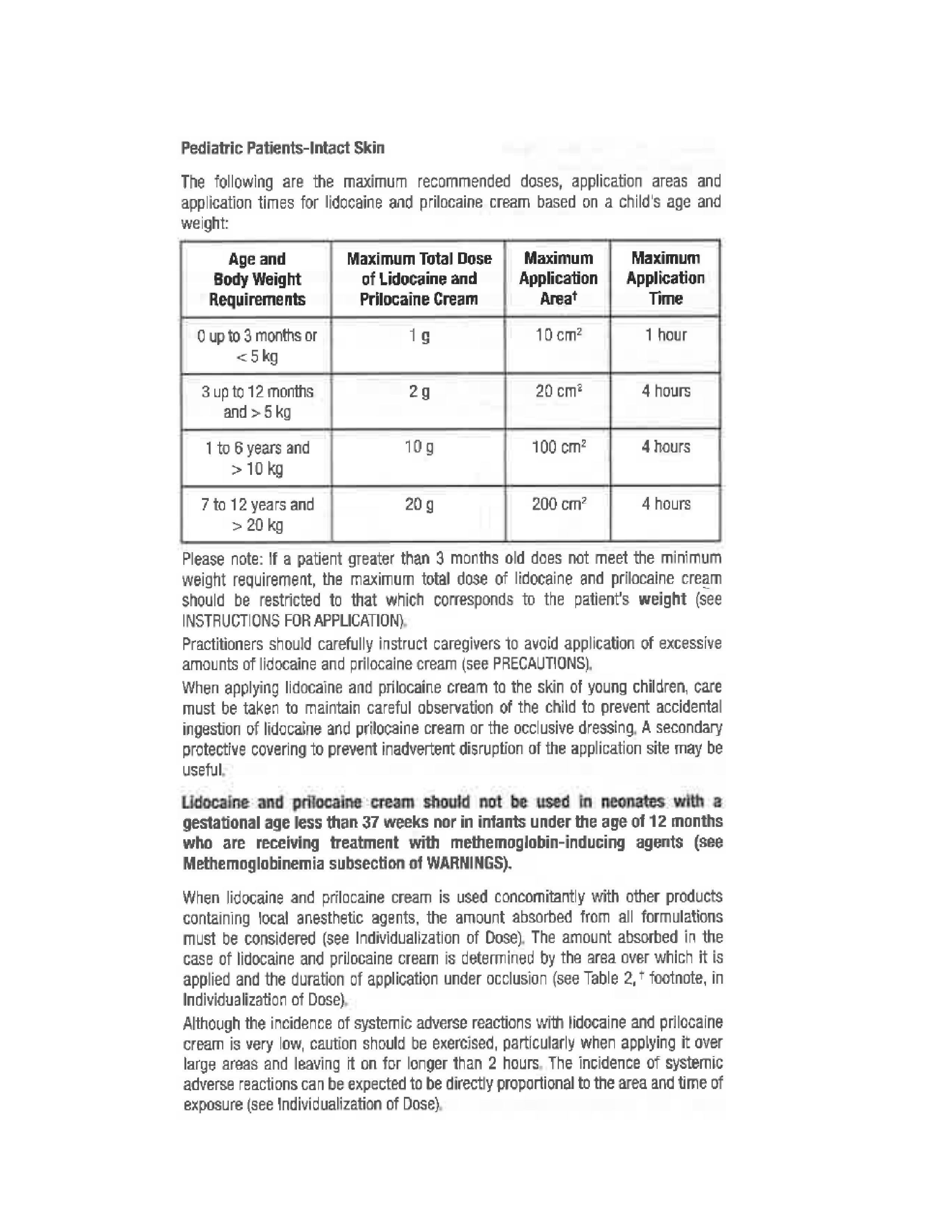
- Pediatric Patients-Intact Skin
- Instructions for Application
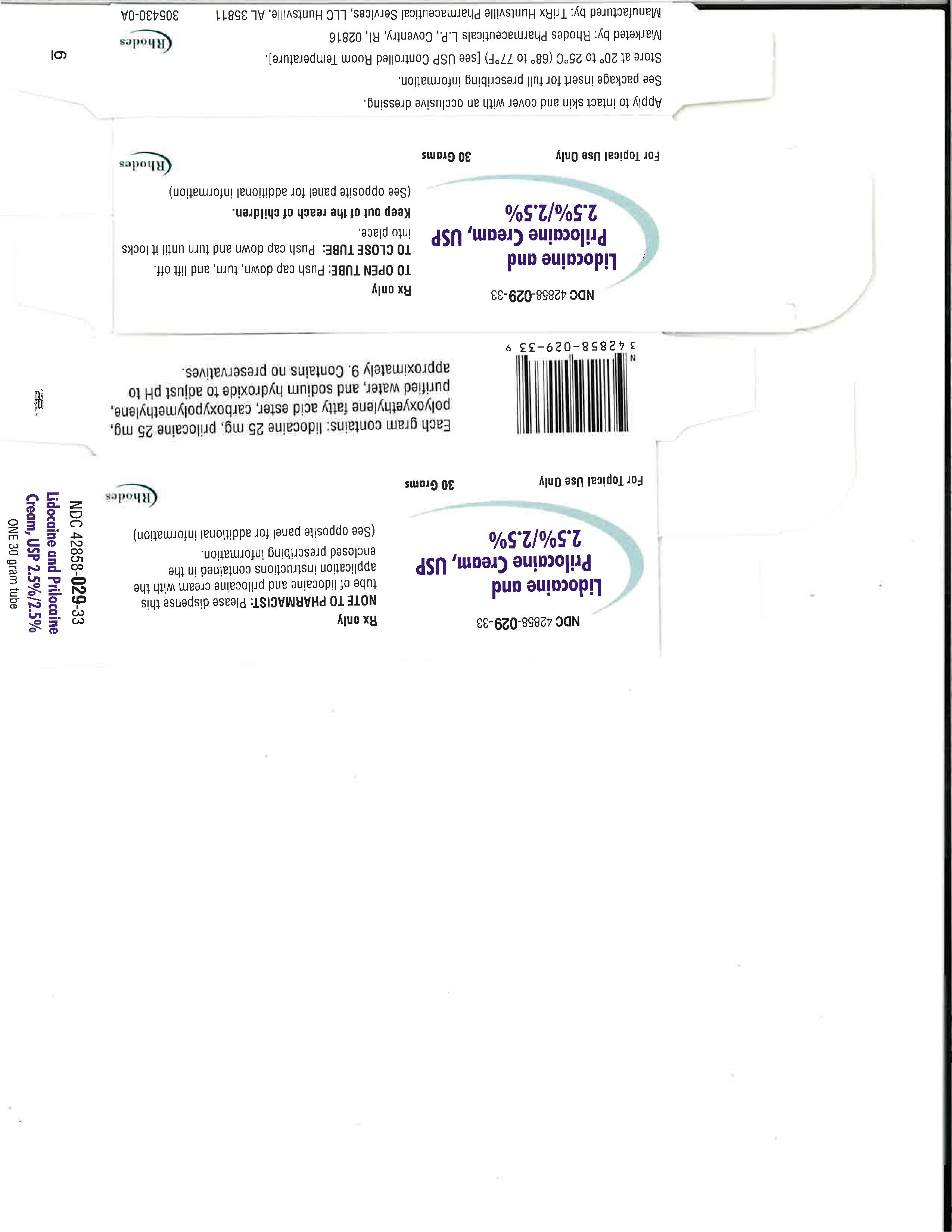
- How Supplied
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- Tube 5g
- Tube 30g
- Carton 5g
- Carton 30g
-
INGREDIENTS AND APPEARANCE
LIDOCAINE AND PRILOCAINE CREAM
lidocaine and prilocaine cream creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:80432-053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 25 mg in 1000 mg PRILOCAINE (UNII: 046O35D44R) (PRILOCAINE - UNII:046O35D44R) PRILOCAINE 25 mg in 1000 mg Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) PEG-54 HYDROGENATED CASTOR OIL (UNII: 0WZF1506N9) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80432-053-65 5000 mg in 1 TUBE; Type 0: Not a Combination Product 08/14/2023 2 NDC:80432-053-71 30000 mg in 1 TUBE; Type 0: Not a Combination Product 08/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213253 08/14/2023 Labeler - TriRx Huntsville Pharmaceutical Services (117090286)