Label: NALOXONE HYDROCHLORIDE spray
- NDC Code(s): 51662-1659-1, 51662-1659-2
- Packager: HF Acquisition Co LLC, DBA HealthFirst
- This is a repackaged label.
- Source NDC Code(s): 45802-578
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (IN EACH SPRAY)
- PURPOSE
- USES
- DIRECTIONS
- WARNINGS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS?
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
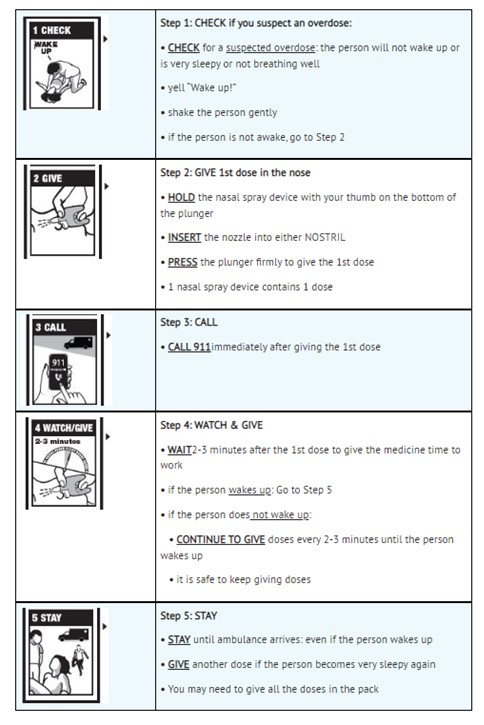
Step 1: CHECK if you suspect an overdose:
• CHECK for a suspected overdose: the person will not wake up or is very sleepy or not breathing well
• yell “Wake up!”
• shake the person gently
• if the person is not awake, go to Step 2
Step 2: GIVE 1st dose in the nose
• HOLD the nasal spray device with your thumb on the bottom of the plunger
• INSERT the nozzle into either NOSTRIL
• PRESS the plunger firmly to give the 1st dose
• 1 nasal spray device contains 1 dose
Step 3: CALL
• CALL 911immediately after giving the 1st dose
Step 4: WATCH & GIVE
• WAIT2-3 minutes after the 1st dose to give the medicine time to work
• if the person wakes up: Go to Step 5
• if the person does not wake up:
• CONTINUE TO GIVE doses every 2-3 minutes until the person wakes up
• it is safe to keep giving doses
Step 5: STAY
• STAY until ambulance arrives: even if the person wakes up
• GIVE another dose if the person becomes very sleepy again
• You may need to give all the doses in the pack
- INDICATIONS & USAGE
- NDC 51662-1659-1
- NDC 51662-1659-2
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51662-1659(NDC:45802-578) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51662-1659-2 2 in 1 CARTON 07/30/2023 1 NDC:51662-1659-1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211951 07/30/2023 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1659)