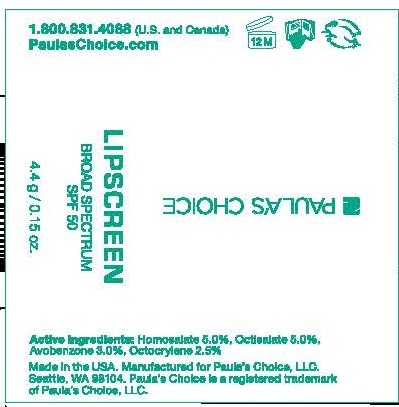
Label: PAULAS CHOICE LIPSCREEN SPF 50- homosalate, octisalate, avobenzone, octocrylene lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 76144-256-01 - Packager: Paula's Choice, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- STOP USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Hydrogenated Soybean Oil, Bis-Diglyceryl Poluacyladipate-2, Hydrogenated Olive Oil, Polyglyceryl-3 Diisostearate, Beeswax, Ozokerite, Hydrogenated Jojoba Oil, Polyethylene, Hydrogenated Polycyclopentadiena, Microcrystalline Wax, Theobrome Cacao (Cocoa) Seed Butter, Silica, Tocopheryl Acetate, Phytosterols, Olea Europaea (Olive) Fruite Oil, Hydrogenated Vegetable Oil, Copernicia Cerifera (Caranauba) Wax, Water (Aqua), Phenoxyethanol.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAULAS CHOICE LIPSCREEN SPF 50
homosalate, octisalate, avobenzone, octocrylene lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76144-256 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 50 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) HYDROGENATED OLIVE OIL (UNII: 53839415GI) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) YELLOW WAX (UNII: 2ZA36H0S2V) CERESIN (UNII: Q1LS2UJO3A) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) COCOA BUTTER (UNII: 512OYT1CRR) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SHEANUT OIL (UNII: O88E196QRF) TOCOPHEROL (UNII: R0ZB2556P8) OLIVE OIL (UNII: 6UYK2W1W1E) CARNAUBA WAX (UNII: R12CBM0EIZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76144-256-01 4.15393 mL in 1 TUBE; Type 0: Not a Combination Product 10/03/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/03/2014 Labeler - Paula's Choice, LLC (029583981)