Label: PAULAS CHOICE CLEAR DAILY SKIN CLEARING TREATMENT- benzoyl peroxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 76144-611-01, 76144-611-02, 76144-611-03 - Packager: Paula's Choice, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 6, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
After cleansing with Paula's Choice CLEAR Pore Normalizing Cleanser and exfoliating with Anti-Redness Exfoliating Solution, cover the affected area with a thin layer 1 to 3 times a day. Because excessive drying of the skin may occur, start with 1 appliation daily and then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. If going outside, use a sunscreen. If sensitivity develops, discontinue use.
- WARNINGS
- DO NOT USE
- ASK DOCTOR
-
WHEN USING
· Avoid unnecessary sun exposure and use a sunscreen · Avoid contact with eyes, lips and mouthIf contact occure, rinse with water · This product may bleach hair or dyed fabrics · Using other topical acne products at the sam etime or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one drug should be used unless direct by a doctor.
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
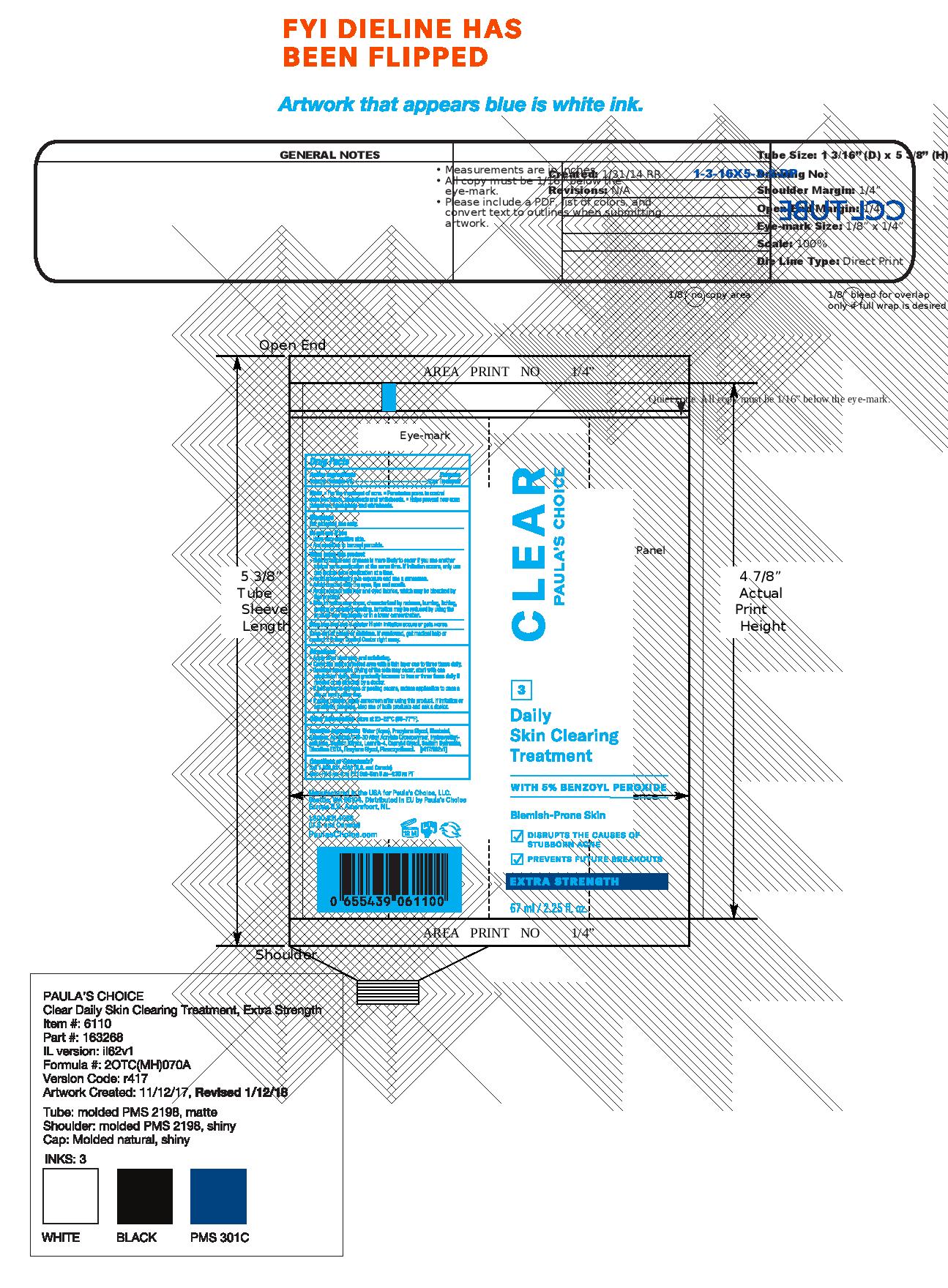
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PAULAS CHOICE CLEAR DAILY SKIN CLEARING TREATMENT
benzoyl peroxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76144-611 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength LEVOMENOL (UNII: 24WE03BX2T) ALLANTOIN (UNII: 344S277G0Z) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) SODIUM CITRATE (UNII: 1Q73Q2JULR) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) HYDROXYETHYL CELLULOSE (5000 CPS AT 1%) (UNII: X70SE62ZAR) LAURETH-4 (UNII: 6HQ855798J) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76144-611-01 67 mL in 1 TUBE; Type 0: Not a Combination Product 09/07/2012 2 NDC:76144-611-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 09/07/2012 3 NDC:76144-611-03 2 mL in 1 PACKET; Type 0: Not a Combination Product 09/07/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 09/07/2012 Labeler - Paula's Choice, LLC (029583981) Registrant - Paula's Choice, LLC (029583981) Establishment Name Address ID/FEI Business Operations Thibiant International, Inc. 083913913 manufacture(76144-611)