Label: NUTRILIPID I.V. FAT EMULSION- soybean oil injection, solution
- NDC Code(s): 0264-4460-00, 0264-4460-10, 0264-4460-20, 0264-4460-30
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NUTRILIPID 20% safely and effectively. See full prescribing information for NUTRILIPID 20%.
NUTRILIPID® 20% (lipid injectable emulsion), for intravenous use
Initial U.S. Approval: 1975RECENT MAJOR CHANGES
Warnings and Precautions (5.3) 7/2025
INDICATIONS AND USAGE
Nutrilipid 20% is indicated as a source of calories and essential fatty acids for parenteral nutrition and as a source of essential fatty acids when a deficiency occurs when oral or enteral nutrition is not possible, insufficient, or contraindicated. (1)
DOSAGE AND ADMINISTRATION
- Nutrilipid 20% Pharmacy Bulk Package is not intended for direct intravenous administration. (2.1)
- For intravenous infusion through a peripheral or central line (2.1)
- Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and consideration of additional energy given to the patient. (2.4)
- For information on the age-appropriate infusion rate, see the full prescribing information. (2.4, 5.1)
Age
Nutritional Requirements Initial Recommended Dosage Maximum Dosage Preterm and term infants (<1 year) 1 to 2 g/kg/day 3 g/kg/day Pediatric patients 1 to 10 years Pediatric patients 11 to <17 years 1 g/kg/day 2.5 g/kg/day Adults 1 to 1.5 g/kg/day 2.5 g/kg/day
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. (5.1, 8.4)
- Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur, consider discontinuation or dosage reduction. (5.2, 8.4)
- Hypersensitivity reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur. (5.3)
- Infections, Fat Overload Syndrome, and Refeeding Syndrome, Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters. (5.4, 5.5, 5.6, 5.7)
- Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates. (5.8, 8.4)
ADVERSE REACTIONS
Adverse reactions include administration site reactions (e.g., erythema, extravasation, pain, phlebitis, pruritus, swelling), hyperlipidemia, hypercoagulability, thrombophlebitis, thrombocytopenia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact B. Braun Medical Inc. at 1-833-425-1464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Coumarin and coumarin derivatives, including warfarin: Anticoagulant activity may be counteracted; monitor laboratory parameters (7.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
2.2 Preparation Instructions for Nutrilipid 20% Bags for Direct Infusion
2.3 Preparation Instructions for Admixing Using Total Parenteral Nutrition Pooling Bags
2.4 Dosing Considerations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants
5.2 Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders
5.3 Hypersensitivity Reactions
5.4 Infections
5.5 Fat Overload Syndrome
5.6 Refeeding Syndrome
5.7 Hypertriglyceridemia
5.8 Aluminum Toxicity
5.9 Monitoring / Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Coumarin and Coumarin Derivatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
- Nutrilipid 20% Pharmacy Bulk Package is not intended for direct intravenous administration.
- Nutrilipid 20% is for intravenous infusion through a peripheral or central line. When administered with dextrose and amino acids, the choice of a central or peripheral venous route should depend on the osmolarity of the final infusate.
- Do not exceed the recommended maximum infusion rate in Table 1 [see Dosage and Administration (2.3) and Warnings and Precautions (5.1)].
- Use a non-vented infusion set or close the air vent on a vented set. Use of a vented intravenous administration set with the vent in the open position could result in air embolism.
- Use a dedicated line without any connections. Multiple connections could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
- Use a 1.2 micron in-line filter.
- Nutrilipid 20% can be infused concurrently into the same vein as carbohydrate-amino acid solutions by means of a Y-connector located near the infusion site; flow rates of each solution should be controlled separately by infusion pumps.
- Do not use administration sets and lines that contain di-2-ethylhexyl phthalate (DEHP). Conventional administration sets contain polyvinyl chloride (PVC) components that have DEHP as a plasticizer.
2.2 Preparation Instructions for Nutrilipid 20% Bags for Direct Infusion
Caution: Nutrilipid 20% Pharmacy Bulk Package is not intended for direct intravenous administration.
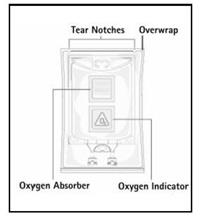
Step 1: Inspect infusion bag overwrap and primary bag and do not use if damaged. Inspect oxygen indicator and do not use if oxygen indicator is pink or dark pink. Use only if container and seals are intact.
Step 2: To open, tear overwrap starting from the tear notches (Figure 1). Remove Nutrilipid 20% bag from overwrap and discard oxygen indicator, oxygen absorber and overwrap.
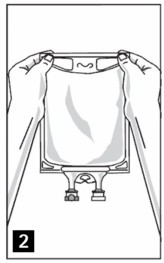
Step 3: Inspect Nutrilipid 20% bag visually (Figure 2). Nutrilipid 20% is a homogenous white and milky, sterile, nonpyrogenic lipid injectable emulsion. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect Nutrilipid 20% to ensure that the emulsion has not separated. Discard the bag if any particulates or discoloration are observed.
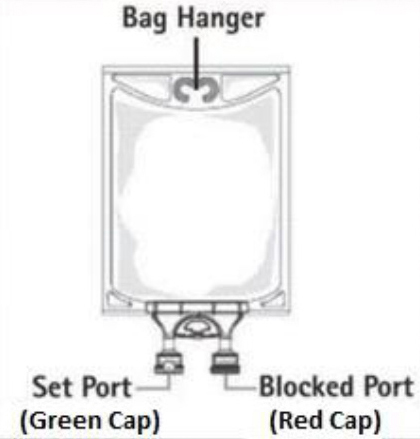
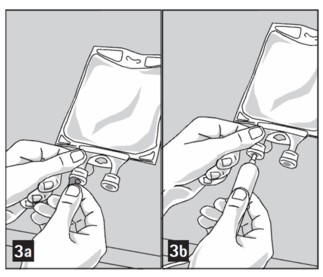
Step 4: Remove aluminum foil of outlet port at the bottom of the bag (Figure 3a) and attach administration set (Figure 3b).
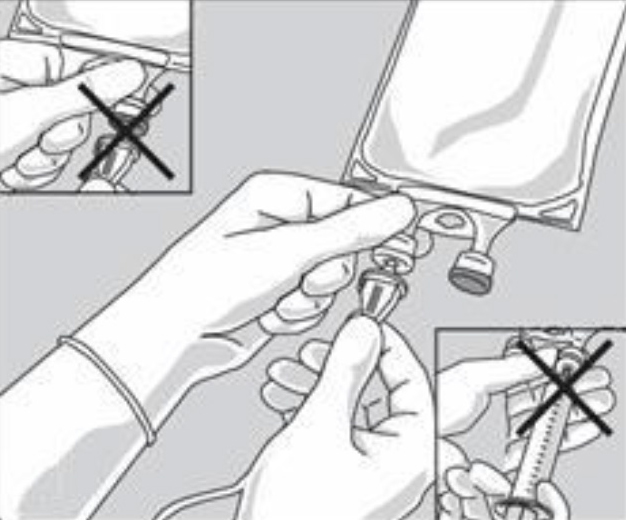
Do not use/penetrate blocked port.
Step 5: Hang bag on IV Pole (Figure 4). If infusion pumps are used, flow rates of each parenteral fluid should be controlled with a separate pump.
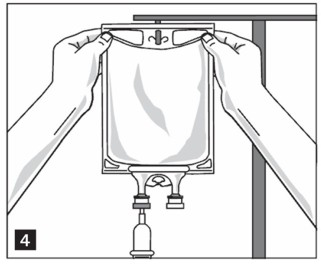
Do not connect flexible bags in series to avoid air embolism due to possible residual gas contained in the primary bag.
Air embolism can result if residual gas in the bag is not fully evacuated prior to administration if the flexible bag is pressurized to increase flow rates.
If administration is controlled by a pumping device, discontinue pumping action before the bag runs dry to avoid air embolism.
2.3 Preparation Instructions for Admixing Using Total Parenteral Nutrition Pooling Bags
- Prepare the admixture into pooling bags using strict aseptic techniques to avoid microbial contamination.
- Do not add additives directly to Nutrilipid 20% Pharmacy Bulk Package.
- Some additives may be incompatible and should not be used. If it is deemed advisable to introduce additives, prepare the admixture using strict aseptic techniques to avoid microbial contamination. Additions to the pooling bag should be evaluated by a pharmacist for compatibility. Questions about compatibility may be directed to B. Braun Medical Inc., Medical Affairs.
- Do not add Nutrilipid 20% to the pooling bag first; destabilization of the lipid may occur from such an admixture.
- The following proper mixing sequence must be followed to minimize pH related problems by ensuring that typically acidic Dextrose Injections are not mixed with lipid emulsions alone:
Manual Admixing
- Manually transfer Dextrose Injection to the Total Parental Nutrition Admixture Container
- Manually transfer Amino Acid Injection
- Manually transfer Nutrilipid 20%
Use gentle agitation during admixing to minimize localized concentration effects; shake bags gently after each addition.
Automated Device Admixing
When admixing parenteral nutrition using an automated device, the Nutrilipid 20% must be separated from the dextrose product by an amino acid product or other non-acidic products.
- The prime destabilizers of emulsions are excessive acidity (such as pH below 5) and inappropriate electrolyte content. Give careful consideration to additions of divalent cations (Ca++ and Mg++), which have been shown to cause emulsion instability. Amino acid solutions exert buffering effects that protect the emulsion.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect Nutrilipid 20% to ensure that:
- precipitates have not formed during the mixing or addition of additives and
- the emulsion has not separated. Separation of the emulsion can be visibly identified by a yellowish streaking or the accumulation of yellowish droplets in the admixed emulsion.
Discard the admixture if any of the above are observed.
- If using an Automated Device, follow the manufacturer instructions.
- Use of the Pharmacy Bulk Package for admixing should be limited to up to four hours after opening.
- Admixtures should be used promptly with storage under refrigeration [2°C to 8°C (36°F to 46°F)] not to exceed 24 hours and must be completely used within 24 hours after removal from refrigeration.
- Do not connect flexible bags in series to avoid air embolism due to possible residual gas contained in the primary bag.
- Air embolism can result if residual gas in the bag is not fully evacuated prior to administration if the flexible bag is pressurized to increase flow rates.
- If administration is controlled by a pumping device, discontinue pumping action before the bag runs dry to avoid air embolism.
- Protect the admixed parenteral nutrition solution from light.
2.4 Dosing Considerations
The dosing of Nutrilipid 20% depends on the patient’s individual energy requirements, influenced by body weight, tolerance, clinical status, age-related growth rate in pediatric patients and the ability to eliminate and metabolize fat.
For partial parenteral nutrition, energy supply by oral or enteral nutrition has to be taken into account. For complete parenteral nutrition, concomitant supplementation with amino acids, carbohydrates, electrolytes, vitamins, and trace elements is necessary.
Prior to administration of Nutrilipid 20%, correct severe water and electrolyte disorders, severe fluid overload states, and severe metabolic disorders. Before starting the infusion, obtain serum triglyceride levels to establish the baseline value.
Recommended Adult and Pediatric Dosing
The recommended nutritional requirements of lipid and recommended dosages of Nutrilipid 20% to be administered to meet those requirements for adults and pediatric patients are provided in Table 1, along with recommendations for the initial and maximum infusion rates. The recommended duration of infusion for Nutrilipid 20% is between 12 and 24 hours, depending on the clinical situation. Adjust the administration flow rate by taking into account the dose being administered, the daily volume/intake, and the duration of the infusion [see Overdosage (10)].
Treatment with parenteral nutrition may be continued for as long as is required by the patient’s condition.
In patients with elevated triglyceride levels, initiate Nutrilipid 20% injection at a lower dose, and advance in smaller increments, monitoring the triglyceride levels with each adjustment [see Warnings and Precautions (5.7) and (5.9)].
When Nutrilipid 20% is administered to correct essential fatty acid deficiency, 8% to 10% of the caloric input should be supplied by Nutrilipid 20% in order to provide adequate amounts of linoleic and linolenic acids.
Table 1: Recommended Pediatric and Adult Dosage and Infusion Rate
Age Nutritional Requirements Direct Infusion Rate* Recommended Initial Dosage and Maximum Dosage Initial Maximum Preterm and term infants (less than 1 year of age)
Initial 1 to 2 g/kg/day
not to exceed 3 g/kg/day†
0.05 mL/min for the first 10 to 15 minutes; gradually increase to the required rate after 15 minutes 0.75 mL/kg/hour Pediatric patients 1 to 10 years of age Initial 1 to 2 g/kg/day
not to exceed 3 g/kg/day†
0.75 mL/kg/hour Pediatric patients 11 to <17 years of age Initial 1 g/kg/day
not to exceed 2.5 g/kg/day†
0.5 mL/kg/hour Adults 1 to 1.5 g/kg/day
not to exceed 2.5 g/kg/day†
0.5 mL/min for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes 0.5 mL/kg/hour -
3 DOSAGE FORMS AND STRENGTHS
Nutrilipid 20% is a homogenous white and milky, sterile, nonpyrogenic lipid injectable emulsion supplied as:
- 20% (50 g/250 mL) (0.2 g/mL) of lipid in 250 mL single-dose flexible bag
- 20% (100 g/500 mL) (0.2 g/mL) of lipid in 500 mL single-dose flexible bag
- 20% (200 g/1,000 mL) (0.2 g/mL) of lipid in 1,000 mL Pharmacy Bulk Package
-
4 CONTRAINDICATIONS
Nutrilipid 20% injection is contraindicated in patients who have:
- Known hypersensitivity to egg, soybean, peanut, or any of the active or inactive ingredients in Nutrilipid 20% [see Warnings and Precautions (5.3)].
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride >1,000 mg/dL) [see Warnings and Precautions (5.7)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants
In the postmarketing setting, serious adverse reactions including acute respiratory distress, metabolic acidosis, and death have been reported in neonates and infants after rapid infusion of intravenous lipid emulsions. Hypertriglyceridemia was commonly reported.
Strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.75 mL/kg/hour [see Dosage and Administration (2.4)].
Preterm and small for gestational age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation [see Warnings and Precautions (5.5, 5.7) and Overdosage (10)].
5.2 Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders
Risk of Parenteral Nutrition-Associated Liver Disease
Parenteral nutrition-associated liver disease (PNALD), also referred to as intestinal failure-associated liver disease (IFALD), can present as cholestasis or hepatic steatosis, and may progress to steatohepatitis with fibrosis and cirrhosis (possibly leading to chronic hepatic failure). The etiology of PNALD is multifactorial; however, intravenously administered phytosterols (plant sterols) contained in plant-derived lipid emulsions, including Nutrilipid 20%, have been associated with development of PNALD.
Monitor liver tests in patients treated with Nutrilipid 20% and consider discontinuation or dosage reduction if abnormalities occur.
Other Hepatobiliary Disorders
Hepatobiliary disorders including cholecystitis and cholelithiasis have developed in some PN-treated patients without preexisting liver disease.
Monitor liver tests when administering Nutrilipid 20%. Patients developing signs of hepatobiliary disorders should be assessed early to determine whether these conditions are related to Nutrilipid 20% use.
5.3 Hypersensitivity Reactions
Nutrilipid 20% contains soybean oil and egg phospholipids which may cause hypersensitivity reactions. Cross reactions have been observed between soybean and peanut. In postmarketing experience, anaphylaxis has been reported following Nutrilipid administration [see Adverse Reactions (6.2)].
Nutrilipid 20% is contraindicated in patients with known hypersensitivity to egg, soybean, peanut, or any of the active or inactive ingredients in Nutrilipid 20% [see Contraindications (4)]. If a hypersensitivity reaction occurs, stop infusion of Nutrilipid 20% immediately and initiate appropriate treatment and supportive measures.
5.4 Infections
Lipid emulsions, such as Nutrilipid 20%, can support microbial growth and are an independent risk factor for the development of catheter-related bloodstream infections. To decrease the risk of infectious complications, ensure aseptic techniques are used for catheter placement, catheter maintenance, and preparation and administration of Nutrilipid 20%.
Monitor for signs and symptoms of infection, including fever and chills, as well as including laboratory test results (including leukocytosis and hyperglycemia). Perform frequent checks of the intravenous catheter insertion site for edema, redness, and discharge.
5.5 Fat Overload Syndrome
Fat overload syndrome is a rare condition that has been reported with intravenous lipid formulations and is characterized by a sudden deterioration in the patient's condition (e.g., fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, hepatomegaly, deteriorating liver function, and central nervous system manifestations such as coma). A reduced or limited ability to metabolize lipids, accompanied by prolonged plasma clearance (resulting in higher lipid levels), may result in this syndrome. Although fat overload syndrome has been most frequently observed when the recommended lipid dose or infusion rate was exceeded, cases have also been described when the lipid formulation was administered according to instructions.
If signs or symptoms of fat overload syndrome occur, stop Nutrilipid 20%. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped.
5.6 Refeeding Syndrome
Administering PN to severely malnourished patients may result in the refeeding syndrome, which is characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. To prevent these complications, closely monitor severely malnourished patients and slowly increase their nutrient intake.
5.7 Hypertriglyceridemia
The use of Nutrilipid 20% is contraindicated in patients with hypertriglyceridemia with serum triglyceride concentrations >1,000 mg/dL.
Patients with conditions such as inherited lipid disorders, obesity, diabetes mellitus, or metabolic syndromes have a higher risk of developing hypertriglyceridemia with the use of Nutrilipid 20%. In addition, patients with hypertriglyceridemia may have worsening of their hypertriglyceridemia with administration of Nutrilipid 20%. Excessive dextrose administration may further increase such risk.
Evaluate patients’ capacity to metabolize and eliminate the infused lipid emulsion by measuring serum triglycerides before the start of infusion (baseline value) and regularly throughout treatment. If triglyceride levels are above 400 mg/dL in adults, stop the Nutrilipid 20% infusion and monitor serum triglyceride levels to avoid clinical consequences of hypertriglyceridemia such as pancreatitis. In pediatric patients with hypertriglyceridemia, lower triglyceride levels (i.e., below 400 mg/dL) may be associated with adverse reactions. Monitor serum triglyceride levels to avoid potential complications with hypertriglyceridemia such as pancreatitis, lipid pneumonitis, and neurologic changes, including kernicterus.
To minimize the risk of new or worsening of hypertriglyceridemia, assess high-risk patients for their overall energy intake including other sources of lipids and dextrose, as well as concomitant drugs that may affect lipid and dextrose metabolism.
5.8 Aluminum Toxicity
Nutrilipid 20% contains no more than 25 mcg/L of aluminum.
Prolonged parenteral nutrition administration in patients with renal impairment may result in aluminum reaching toxic levels. Preterm infants are at greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions that contain aluminum.
Patients with impaired kidney function, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration of total parenteral nutrition products.
5.9 Monitoring / Laboratory Tests
Monitor fluid status closely in patients with pulmonary edema or heart failure.
Throughout treatment, monitor serum triglycerides [see Warnings and Precautions (5.7)], fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count (including platelets), and coagulation parameters.
The lipids contained in Nutrilipid may interfere with some laboratory tests (e.g., hemoglobin, lactate dehydrogenase, bilirubin, oxygen saturation) if blood is sampled before the lipids have cleared from the bloodstream. Conduct these tests at least 6 hours after stopping the infusion.
Nutrilipid contains Vitamin K that may counteract anticoagulant activity [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
Adverse Reactions described elsewhere in labeling:
- Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants [see Warnings and Precautions (5.1)]
- Risk of Parenteral Nutrition Associated Liver Disease [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Infections [see Warnings and Precautions (5.4)]
- Fat Overload Syndrome [see Warnings and Precautions (5.5)]
- Refeeding Syndrome [see Warnings and Precautions (5.6)]
- Hypertriglyceridemia [see Warnings and Precautions (5.7)]
- Aluminum Toxicity [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported with other intravenous lipid emulsions include hyperlipidemia, hypercoagulability, thrombophlebitis, and thrombocytopenia.
Adverse reactions reported in long-term use with other intravenous lipid emulsions include hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, abnormalities in liver function tests, brown pigmentation of the liver and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Nutrilipid 20%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General disorders and administration site conditions: extravasation, infusion site erythema, infusion site pain, infusion site phlebitis, infusion site pruritus, and infusion site swelling.
Immune system disorders: hypersensitivity reactions, including anaphylaxis [see Contraindications (4), Warnings and Precautions (5.3)].
-
7 DRUG INTERACTIONS
7.1 Coumarin and Coumarin Derivatives
The soybean oil in Nutrilipid 20% contains vitamin K1. Vitamin K can reverse the anticoagulant activity of coumarin and coumarin derivatives, including warfarin, which work by blocking recycling of vitamin K. Monitor laboratory parameters for anticoagulant activity in patients who are on both Nutrilipid 20% and coumarin or coumarin derivatives.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate or well controlled studies with Nutrilipid 20% in pregnant women. Additionally, animal reproduction studies have not been conducted with Nutrilipid 20%. It is not known whether Nutrilipid 20% can cause fetal harm when administered to a pregnant woman. Nutrilipid 20% should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether Nutrilipid 20% is present in human milk. Because many drugs are present in human milk, caution should be exercised when Nutrilipid 20% is administered to a nursing woman.
8.4 Pediatric Use
The evidence for safety and efficacy in pediatric patients of Nutrilipid 20% as a source of calories and essential fatty acids for parenteral nutrition and as a source of essential fatty acids when a deficiency occurs when oral or enteral nutrition is not possible, insufficient, or contraindicated is derived from the published literature and clinical experience with similar soybean oil-based intravenous lipid emulsions.
In the postmarketing setting, clinical decompensation with rapid infusion of intravenous lipid emulsion in neonates and infants, sometimes fatal, has been reported [see Warnings and Precautions (5.1)]. Patients, particularly preterm infants, are at risk for aluminum toxicity [see Warnings and Precautions (5.9)]. Patients, including pediatric patients, may be at risk for PNALD [see Warnings and Precautions (5.2)]. In clinical trials of a pure soybean oil based intravenous lipid emulsion product, thrombocytopenia in neonates occurred (less than 1%).
8.5 Geriatric Use
Clinical studies of Nutrilipid 20% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
In the event of overdose, serious adverse reactions may result [see Warnings and Precautions (5.1, 5.5)]. Stop the infusion to allow lipids to clear from serum. The effects are usually reversible after the lipid infusion is stopped. If medically appropriate, further intervention may be indicated. The lipid administered and fatty acids produced are not dialyzable.
-
11 DESCRIPTION
Nutrilipid 20% is a homogenous white and milky sterile, nonpyrogenic lipid emulsion for intravenous administration.
Each 100 mL of Nutrilipid 20% contains: Soybean Oil 20 g; Egg Yolk Phospholipid 1.2 g; Glycerin USP (glycerol) 2.5 g; Sodium Oleate 0.03 g; Water for Injection USP qs.
pH adjusted with Sodium Hydroxide NF.
pH: 6.8 (6.0-8.9); Osmolality: 390 mOsmol/kg (actual). Contains emulsified fat particles averaging approximately 0.26 micron in diameter, similar to naturally occurring chylomicrons. The total caloric value, including fat, phospholipid, and glycerol is 2.0 Kcal per mL.
Soybean oil is a refined natural product consisting of a mixture of neutral triglycerides of predominantly unsaturated fatty acids with the following structure:
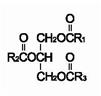
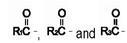 are saturated and unsaturated fatty acid residues. The major component fatty acids are linoleic (48% - 58%), oleic (17% - 30%), palmitic (9% -13%), linolenic (4% - 11%), and stearic (2.5% - 5.0%). These fatty acids have the folIowing chemical and structural formulas:
are saturated and unsaturated fatty acid residues. The major component fatty acids are linoleic (48% - 58%), oleic (17% - 30%), palmitic (9% -13%), linolenic (4% - 11%), and stearic (2.5% - 5.0%). These fatty acids have the folIowing chemical and structural formulas:Linoleic Acid
C18H32O2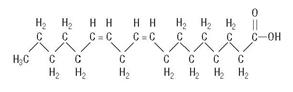
Oleic Acid
C18H34O2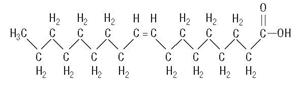
Palmitic Acid
C16H32O2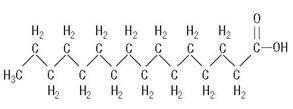
Linolenic Acid
C18H30O2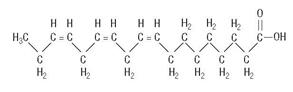
Stearic Acid
C18H36O2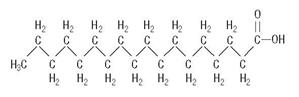
Egg yolk phospholipids are a mixture of naturally occurring phospholipids isolated from egg yolk.
Glycerol is chemically designated C3H8O3 and is a clear colorless, hygroscopic syrupy liquid. It is added to adjust tonicity.
Not made with natural rubber latex, PVC or DEHP.
Drug product contains no more than 25 mcg/L of aluminum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nutrilipid 20% administered intravenously provides biologically utilizable source of calories and essential fatty acids.
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are important for membrane structure and function, precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
Nutrilipid 20% causes an increase in heat production, decrease in respiratory quotient, and increase in oxygen consumption following its administration.
12.3 Pharmacokinetics
The infused lipid particles are removed from the bloodstream in a manner generally thought to be similar to the enzymatic clearance of naturally produced chylomicrons formed after enteral fat intake. Following infusion, there is a transient increase in plasma triglycerides. The triglycerides are hydrolyzed to free fatty acids and glycerol by the enzyme lipoprotein lipase. The free fatty acids either enter the tissues (where they may be oxidized or resynthesized into triglycerides and stored) or circulate in the plasma, bound to albumin. In the liver, circulating free fatty acids are oxidized or converted to very low-density lipoproteins that re-enter the bloodstream.
Phosphatides are the hydrophobic components of membranes and provide electrically insulated layers. They are involved in the formation of membrane structures. Choline prevents deposition of fat in the liver.
Glycerol is metabolized to carbon dioxide and glycogen or is used in the synthesis of body fats.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Nutrilipid 20% is supplied as a homogenous white and milky, sterile, nonpyrogenic, lipid injectable emulsion in the following strengths:
Strengths REF Volume NDC Number
20% (50 g/250 mL) (0.2 g/mL) S4603 250 mL 0264-4460-30 20% (100 g/500 mL) (0.2 g/mL) S4601 500 mL 0264-4460-10 20% (200 g/1,000 mL) (0.2 g/mL) S4600 1,000 mL 0264-4460-00
(Pharmacy Bulk Container)Do not freeze. If accidentally frozen, discard the container.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].
Use of the Pharmacy Bulk Package for admixing should be limited to up to four hours after opening. Admixtures should be used promptly with storage under refrigeration [2°C to 8°C (36°F to 46°F)] not to exceed 24 hours and must be completely used within 24 hours after removal from refrigeration.
-
17 PATIENT COUNSELING INFORMATION
When initiating Nutrilipid 20% administration, discuss the following information with the patient or caregiver:
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants
Inform caregivers that acute respiratory distress and death may occur in neonates and infants after rapid infusion of intravenous lipid emulsions. If Nutrilipid is infused at home, instruct caregivers not to exceed maximum infusion rate [see Warnings and Precautions (5.1)].
Parenteral Nutrition-Associated Liver Disease and Other Hepatobiliary Disorders
Inform patients and caregivers that use of parenteral nutrition may result in parenteral nutrition-associated liver disease and/or other hepatobiliary disorders [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
Inform patients and caregivers that Nutrilipid 20% may cause hypersensitivity reactions, including anaphylaxis. If Nutrilipid 20% is infused at home, instruct patients and caregivers to stop the infusion of Nutrilipid 20% immediately and seek medical attention if they experience signs or symptoms of a hypersensitivity reaction, such as rapid or weak heartbeat, feeling faint, difficulty in breathing or swallowing, vomiting, nausea, headache, sweating, dizziness, hives, rash, itching, flushing, dizziness, fever, or chills [see Warnings and Precautions (5.3)].
Infections
Inform patients and caregivers that patients who receive Nutrilipid 20% are at risk of infection. If Nutrilipid 20% is infused at home, instruct patients and caregivers to ensure aseptic techniques are used for the preparation and administration of Nutrilipid 20% and to monitor for signs and symptoms of infection [see Warnings and Precautions (5.4)].
Fat Overload Syndrome
Inform patients and caregivers that fat overload syndrome has been reported with the use of intravenous lipid emulsions. If Nutrilipid 20% is infused at home, instruct patients and caregivers to stop Nutrilipid 20% if signs or symptoms of fat overload syndrome occur [see Warnings and Precautions (5.5)].
Refeeding Syndrome
If the patient is severely malnourished, inform patients and caregivers that administering parenteral nutrition including Nutrilipid 20% may result in refeeding syndrome [see Warnings and Precautions (5.6)].
Hypertriglyceridemia
Inform patients and caregivers about the risks of hypertriglyceridemia with Nutrilipid 20% use [see Warnings and Precautions (5.7)].
Aluminum Toxicity
Inform patients and caregivers that prolonged PN administration in patients with renal impairment, including preterm neonates, may result in aluminum reaching toxic levels associated with central nervous system and bone toxicity [see Warnings and Precautions (5.8)].
Preparation and Administration Instructions
If it is acceptable for a patient and caregiver to administer Nutrilipid 20% at home, then provide recommendations on how to prepare, administer, and store Nutrilipid 20% [see Dosage and Administration (2.1, 2.2)].
- SPL UNCLASSIFIED SECTION
-
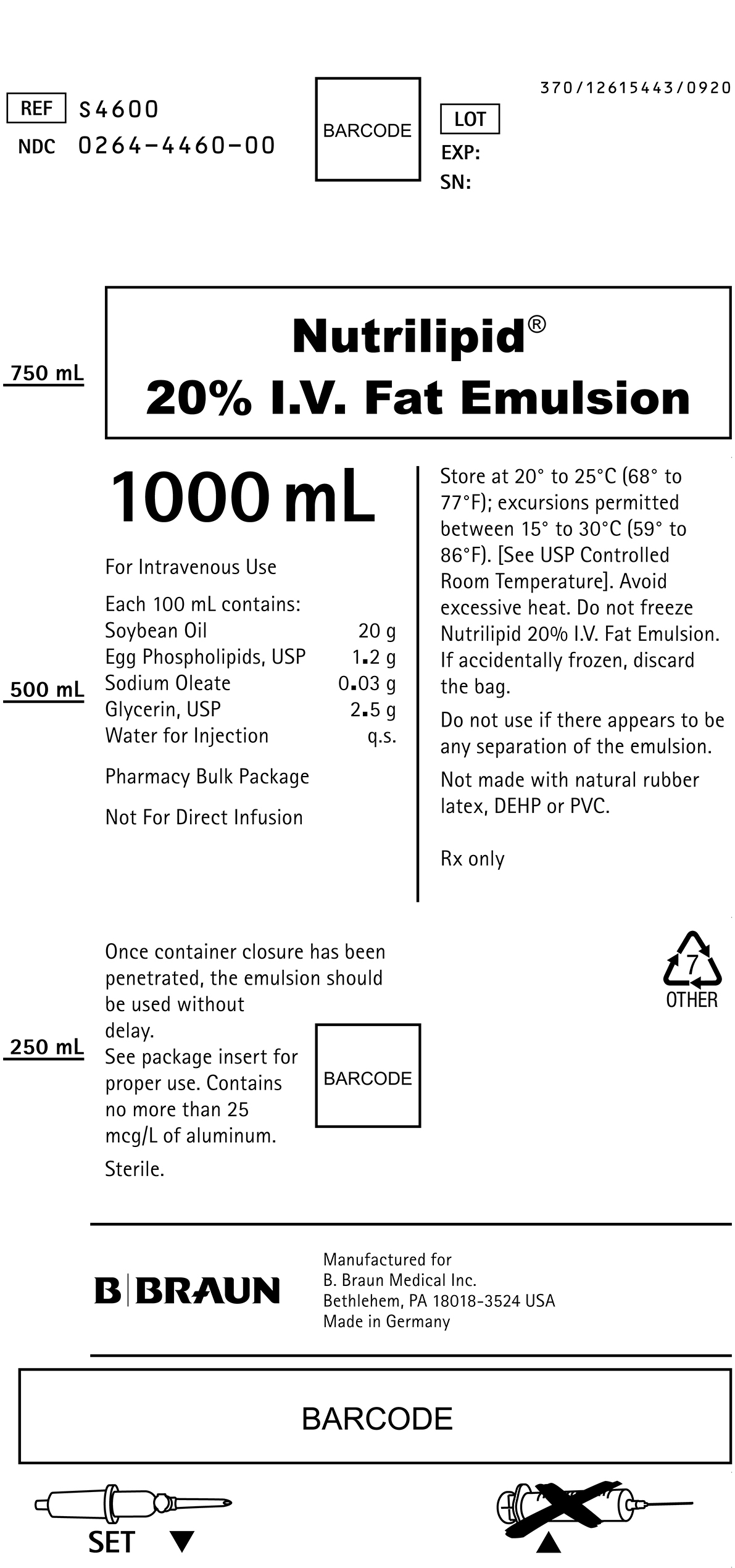
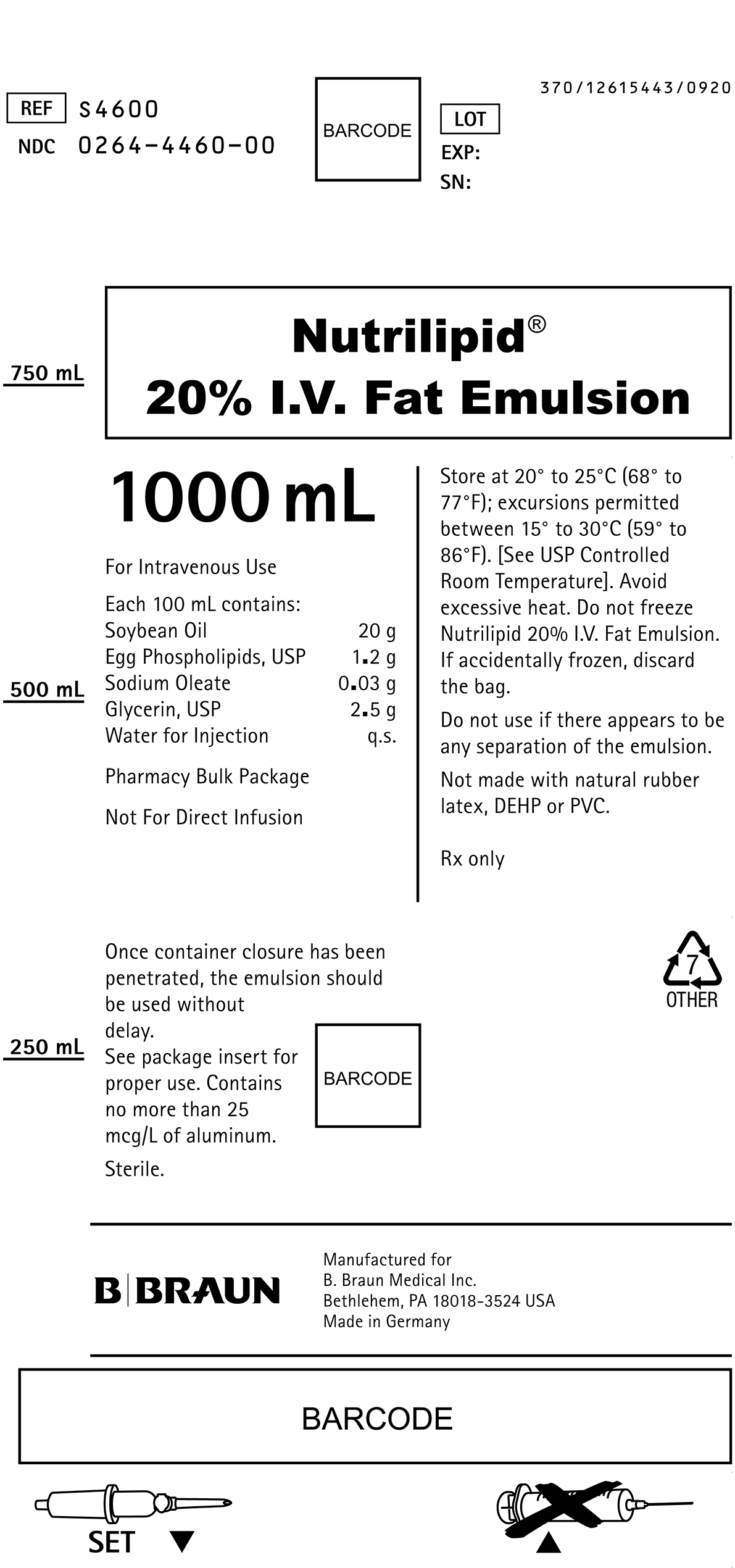
PRINCIPAL DISPLAY PANEL - 1000 mL
REF S4600
NDC 0264-4460-00LOT
EXP:
SN:Nutrilipid®
20% I.V. Fat Emulsion1000 mL
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Pharmacy Bulk Package
Not For Direct Infusion
Once container closure has been penetrated, the emulsion should be used without delay.
See package insert for proper use. Contains no more than 25 mcg/L of aluminum.
Sterile.
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]. Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag.
Do not use if there appears to be any separation of the emulsion.
Not made with natural rubber latex, DEHP or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
370/12615443/0920
-
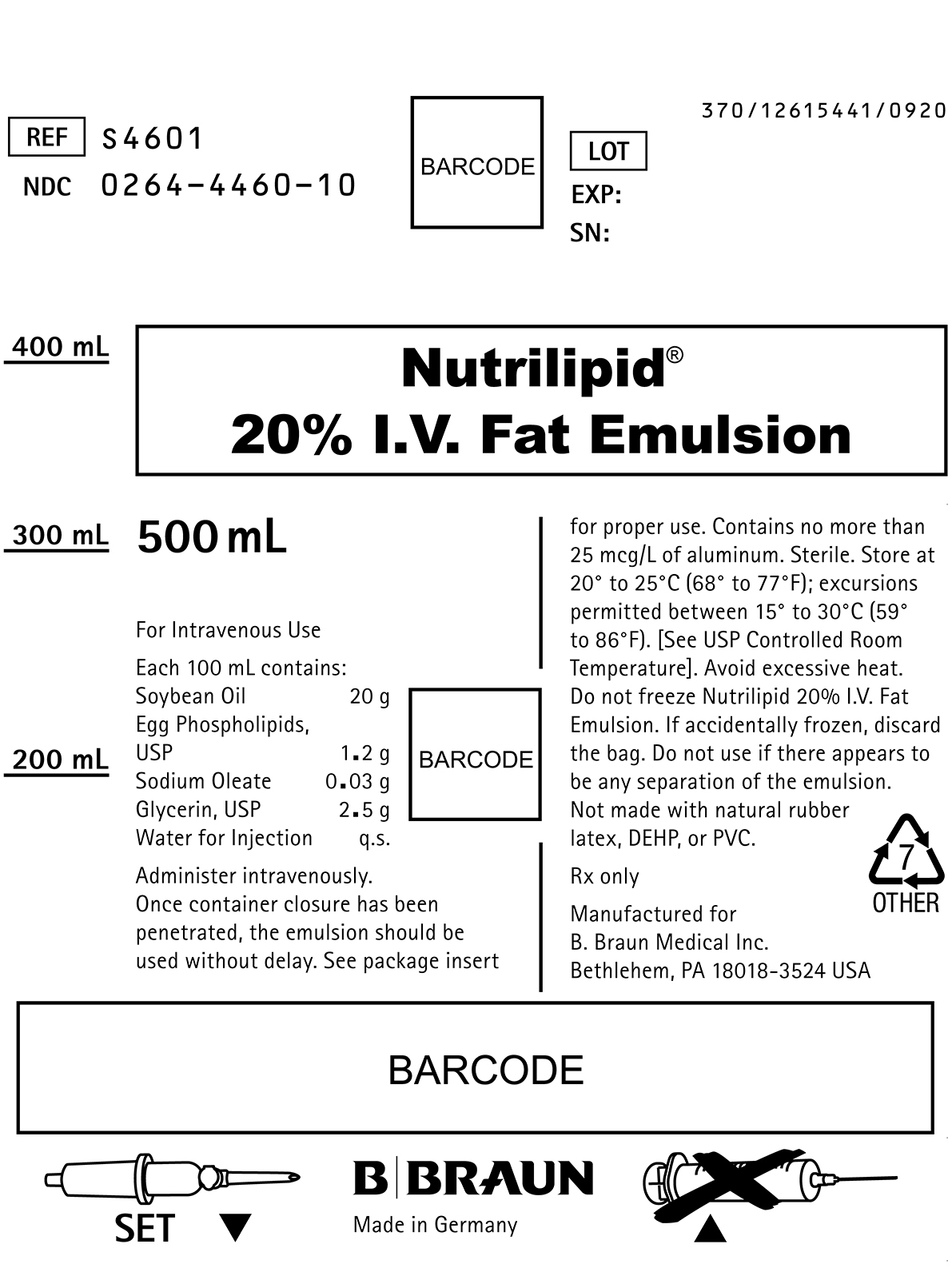
PRINCIPAL DISPLAY PANEL - 500 mL
REF S4601
NDC 0264-4460-10LOT
EXP:
SN:Nutrilipid®
20% I.V. Fat Emulsion500 mL
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Administer intravenously.
Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum. Sterile. Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]. Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag. Do not use if there appears to be any separation of the emulsion.
Not made with natural rubber latex, DEHP, or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
370/12615441/0920
-
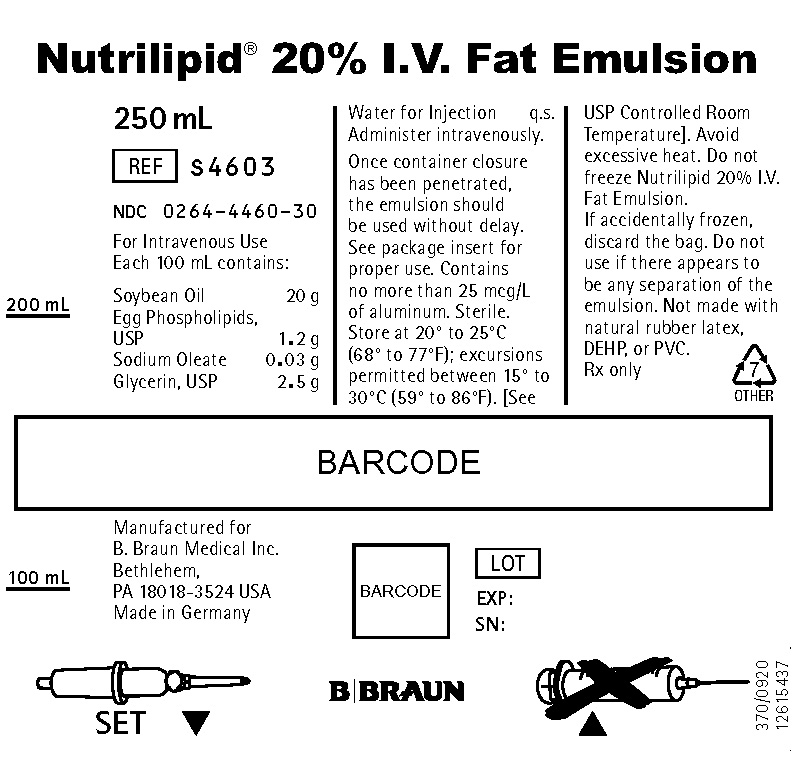
PRINCIPAL DISPLAY PANEL - 250 mL
Nutrilipid® 20% I.V. Fat Emulsion
250 mL
REF S4603NDC 0264-4460-30
For Intravenous Use
Each 100 mL contains:Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.
Administer intravenously.Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum. Sterile. Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]. Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion.
If accidentally frozen, discard the bag. Do not use if there appears to be any separation of the emulsion. Not made with natural rubber latex, DEHP, or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanyLOT
EXP:
SN:SET
370/0920
12615437
-
INGREDIENTS AND APPEARANCE
NUTRILIPID I.V. FAT EMULSION
soybean oil injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-4460 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOYBEAN OIL (UNII: 241ATL177A) (SOYBEAN OIL - UNII:241ATL177A) SOYBEAN OIL 20 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) 1.2 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 2.5 g in 100 mL SODIUM OLEATE (UNII: 399SL044HN) 0.03 g in 100 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-4460-10 12 in 1 CASE 06/30/2014 1 1 in 1 CARTON 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:0264-4460-20 12 in 1 CASE 06/30/2014 03/31/2020 2 1 in 1 CARTON 2 500 mL in 1 CONTAINER; Type 0: Not a Combination Product 3 NDC:0264-4460-30 12 in 1 CASE 06/30/2014 3 1 in 1 CARTON 3 250 mL in 1 CONTAINER; Type 0: Not a Combination Product 4 NDC:0264-4460-00 8 in 1 CASE 06/30/2014 4 1 in 1 CARTON 4 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019531 06/30/2014 Labeler - B. Braun Medical Inc. (002397347)