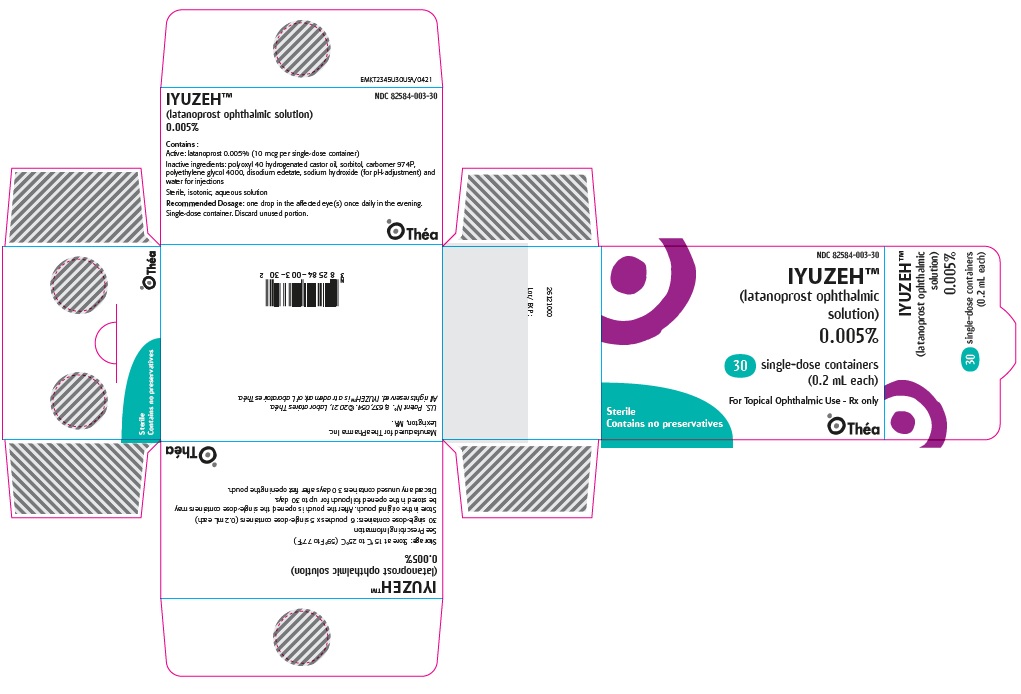
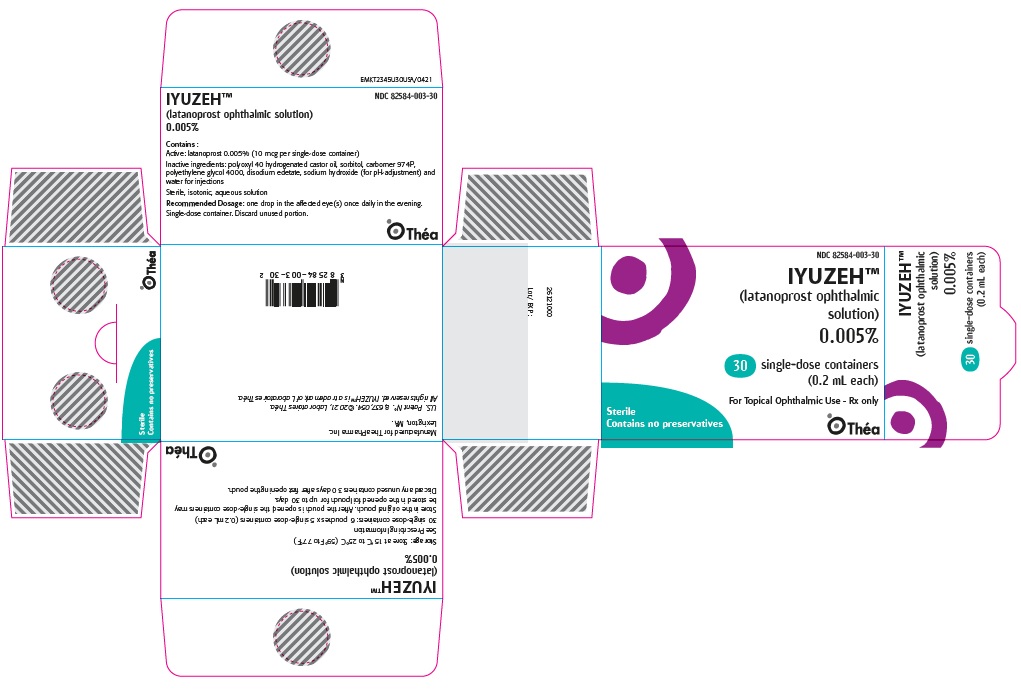
Label: IYUZEH- latanoprost ophthalmic solution 0.005% solution/ drops
- NDC Code(s): 82584-003-30
- Packager: Thea Pharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IYUZEH safely and effectively. See full prescribing information for IYUZEH.
IYUZEH (latanoprost ophthalmic solution) 0.005%, for topical ophthalmic use
Initial U.S. Approval: 1996INDICATIONS AND USAGE
IYUZEH is a prostaglandin F2α analogue indicated for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. ( 1)
DOSAGE AND ADMINISTRATION
One drop in the affected eye(s) once daily in the evening. ( 2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing latanoprost 0.005% (50 mcg/mL). ( 3)
CONTRAINDICATIONS
Known hypersensitivity to latanoprost or any other ingredients in this product. ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (5% to 35%) for IYUZEH are: conjunctival hyperemia, eye irritation, eye pruritus, abnormal sensation in eye, foreign body sensation in eyes, vision blurred and lacrimation increased. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Thea Pharma Inc. at 1-833-838-4028 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 03/2024
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
5.2 Eyelash Changes
5.3 Intraocular Inflammation
5.4 Macular Edema
5.5 Herpetic Keratitis
5.6 Contact Lens Use
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Elevated Baseline IOP
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal.
The dosage of IYUZEH should not exceed once daily; the combined use of two or more prostaglandins, or prostaglandin analogs including IYUZEH is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Reduction of the IOP starts approximately 3 to 4 hours after administration and the maximum effect is reached after 8 to 12 hours.
IYUZEH may be used concomitantly with other topical ophthalmic drug products to lower IOP. In vitro studies have shown that precipitation occurs when eye drops containing thimerosal are mixed with the preserved 0.005% latanoprost reference product. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. Contact lenses should be removed prior to the administration of IYUZEH and may be reinserted 15 minutes after administration.
The solution from one individual unit is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual unit is opened, the remaining contents should be discarded immediately after administration.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Topical latanoprost ophthalmic products, including IYUZEH have been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid), and eyelashes. Pigmentation is expected to increase as long as latanoprost is administered.
The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of latanoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long-term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with IYUZEH can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
5.2 Eyelash Changes
Latanoprost ophthalmic products, including IYUZEH may gradually change eyelashes and vellus hair in the treated eye; these changes include increased length, thickness, pigmentation, the number of lashes or hairs, and misdirected growth of eyelashes. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Intraocular Inflammation
IYUZEH should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation because inflammation may be exacerbated.
5.4 Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with latanoprost ophthalmic products, including IYUZEH. IYUZEH should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
5.5 Herpetic Keratitis
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. IYUZEH should be used with caution in patients with a history of herpetic keratitis. IYUZEH should be avoided in cases of active herpes simplex keratitis because inflammation may be exacerbated.
-
6 ADVERSE REACTIONS
The following adverse reactions have been reported with the use of topical latanoprost products and are discussed in greater detail in other sections of the label:
- Iris pigmentation changes [see Warnings and Precautions (5.1)]
- Eyelid skin darkening [see Warnings and Precautions (5.1)]
- Eyelash changes (increased length, thickness, pigmentation, and number of lashes) [see Warnings and Precautions (5.2)]
- Intraocular inflammation (iritis/uveitis) [see Warnings and Precautions (5.3)]
- Macular edema, including cystoid macular edema [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In the two clinical trials conducted with IYUZEH (latanoprost ophthalmic solution) 0.005% comparing it to XALATAN the preserved 0.005% latanoprost reference product, the most frequently reported ocular adverse reactions were conjunctival hyperemia and eye irritation ( Table 1).
Table 1. Ocular Adverse Reactions Reported by ≥ 1% of Subjects Receiving IYUZEH Symptom/Finding Adverse Reactions (incidence (%)) IYUZEH
(n=378)XALATAN
(n=358)Conjunctival hyperemia 129 (34) 133 (37) Eye irritation 72 (19) 112 (31) Eye pruritus 57 (15) 58 (16) Abnormal sensation in eye 51 (14) 52 (15) Foreign body sensation in eyes 44 (12) 36 (10) Vision blurred 28 (7) 30 (8) Lacrimation increased 19 (5) 14 (4) Photophobia 13 (3) 17 (5) 6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of topical latanoprost products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to ophthalmic latanoprost products, or a combination of these factors, include:
- Nervous System Disorders: Dizziness; headache; toxic epidermal necrolysis
- Eye Disorders: Eyelash and vellus hair changes of the eyelid (increased length, thickness, pigmentation, and number of eyelashes); keratitis; corneal edema and erosions; intraocular inflammation (iritis/uveitis); macular edema, including cystoid macular edema; trichiasis; periorbital and lid changes resulting in deepening of the eyelid sulcus; iris cyst; eyelid skin darkening; localized skin reaction on the eyelids; conjunctivitis; pseudopemphigoid of the ocular conjunctiva.
- Respiratory, Thoracic and Mediastinal Disorders: Asthma and exacerbation of asthma; dyspnea
- Gastrointesting Disorders: Nausea; vomiting
- Skin and Subcutaneous Tissue Disorders: Pruritis
- Infections and Infestations: Herpes keratitis
- Cardiac Disorders: Angina; palpitations; angina unstable
- General Disorders and Administration Site Conditions: Chest pain
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies of IYUZEH administration in pregnant women to inform drug-associated risks.
In animal reproduction studies, intravenous (IV) administration of latanoprost to pregnant rabbits and rats throughout the period of organogenesis produced malformations, embryofetal lethality and spontaneous abortion at clinically relevant doses (equivalent to 1.3 – 324 times the maximum recommended human ophthalmic dose, on a mg/m 2 basis, assuming 100% absorption) (see Data) .
The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk in the U.S. general population of major birth defects is 2 to 4%, and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Animal Data
Embryofetal studies were conducted in pregnant rabbits administered latanoprost daily by IV injection on gestation days 6 through 18, to target the period of organogenesis. A no observed adverse effect level (NOAEL) was not established for rabbit developmental toxicity. Post-implantation loss due to late resorption was shown as doses ≥0.2 mcg/kg/day (equivalent to 1.3 times the maximum recommended human ophthalmic dose [RHOD], on a mg/m 2 basis, assuming 100% absorption). Spina bifida and abortion occurred at 5 mcg/kg/day (equivalent to 32 times the maximum RHOD). Total litter loss due to early resorption was observed at doses ≥50 mcg/kg/day (324 times the maximum RHOD). Transient signs of maternal toxicity were observed after IV dosing (increased breathing, muscle tremors, slight motor incoordination) at 300 mcg/kg/day (1946 times the maximum RHOD). No maternal toxicity was observed at doses up to 50 mcg/kg/day.Embryofetal studies were conducted in pregnant rats administered latanoprost daily by IV injection on gestation days 6 through 15, to target the period of organogenesis. A NOAEL for rat developmental toxicity was not established. Cleft palate was observed at 1 mcg/kg (equivalent to 3.2 times the maximum RHOD, on a mg/m 2 basis, assuming 100% absorption). Brain porencephalic cyst(s) were observed ≥50 mcg/kg (162 times the maximum RHOD). Skeletal anomalies were observed at 250 mcg/kg (811 times the maximum RHOD). No maternal toxicity was detectable at 250 mcg/kg/day.
Prenatal and postnatal development was assessed in rats. Pregnant rats were administered latanoprost daily by IV injection from gestation day 15, through delivery, until weaning (lactation Day 21). No adverse effects on rat offspring were observed at doses up to 10 mcg/kg/day (32 times the maximum RHOD, on a mg/m2 basis, assuming 100% absorption). At 100 mcg/kg/day (324 times the maximum RHOD), maternal deaths and pup mortality occurred.
8.2 Lactation
Risk Summary
It is not known whether this drug or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when IYUZEH is administered to a nursing woman.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IYUZEH and any potential adverse effects on the breastfed child from IYUZEH.
-
10 OVERDOSAGE
Intravenous infusion of up to 3 mcg/kg of latanoprost in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment with latanoprost ophthalmic solution and no adverse reactions were observed. IV dosages of 5.5 to 10 mcg/kg caused abdominal pain, dizziness, fatigue, hot flushes, nausea, and sweating.
-
11 DESCRIPTION
Latanoprost is a prostaglandin F 2α analogue. Its chemical name is isopropyl-(Z)-7[(1R,2R,3R,5S)3,5dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5-heptenoate. Its molecular formula is C 26H 40O 5 and its chemical structure is:
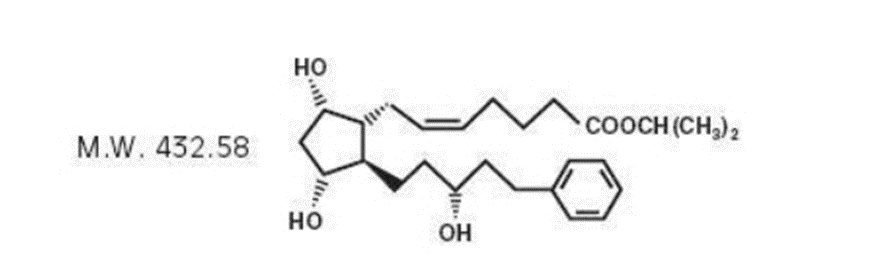
Latanoprost is a colorless to yellow oil that is very soluble in acetonitrile and freely soluble in ethanol, ethyl acetate, and methanol. It is practically insoluble in water and hexanes.
IYUZEH (latanoprost ophthalmic solution) 0.005% is supplied as a sterile, isotonic, aqueous solution of latanoprost with a pH of approximately 7 and an osmolality of approximately 280 mOsmol/kg. Each mL of IYUZEH contains 50 mcg of latanoprost. The inactive ingredients are: polyoxyl 40 hydrogenated castor oil, sorbitol, carbomer 974P, polyethylene glycol 4000, disodium edetate, sodium hydroxide (for pH-adjustment) and water for injections. One drop contains approximately 1.5 mcg of latanoprost.
IYUZEH does not contain a preservative.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Latanoprost is a prostaglandin F 2α analogue that is believed to reduce the IOP by increasing the outflow of aqueous humor. Studies in animals and man suggest that the main mechanism of action is increased uveoscleral outflow. Elevated IOP represents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
12.2 Pharmacodynamics
Reduction of the IOP in man starts about 3-4 hours after administration and maximum effect is reached after 8-12 hours. IOP reduction is present for at least 24 hours.
12.3 Pharmacokinetics
Absorption
Latanoprost is absorbed through the cornea where the isopropyl ester prodrug is hydrolyzed to the acid form to become biologically active.
Distribution
The distribution volume in humans is 0.16 ± 0.02 L/kg. The acid of latanoprost can be measured in aqueous humor during the first 4 hours, and in plasma only during the first hour after local administration. Studies in man indicate that the peak concentration in the aqueous humor is reached about two hours after topical administration.
Elimination
Metabolism
Latanoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to the biologically active acid. The active acid of latanoprost reaching the systemic circulation is primarily metabolized by the liver to the 1,2-dinor and 1,2,3,4-tetranor metabolites via fatty acid β-oxidation.Excretion
The elimination of the acid of latanoprost from human plasma is rapid (t 1/2 = 17 min) after both IV and topical administration. Systemic clearance is approximately 7 mL/min/kg. Following hepatic β-oxidation, the metabolites are mainly eliminated via the kidneys. Approximately 88% and 98% of the administered dose are recovered in the urine after topical and IV dosing, respectively. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Latanoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses of up to 170 mcg/kg/day (approximately 2800 times the recommended maximum human dose) for up to 20 and 24 months, respectively.
Mutagenesis
Latanoprost was not mutagenic in bacteria, in mouse lymphoma, or in mouse micronucleus tests. Chromosome aberrations were observed in vitro with human lymphocytes. Additional in vitro and in vivo studies on unscheduled DNA synthesis in rats were negative.
Impairment of Fertility
Latanoprost has not been found to have any effect on male or female fertility in rat studies at IV doses up to 250 mcg/kg/day (811 times the maximum RHOD, on a mg/m 2 basis, assuming 100% absorption).
-
14 CLINICAL STUDIES
14.1 Elevated Baseline IOP
In randomized, controlled clinical trials of patients with open angle glaucoma or ocular hypertension with mean baseline IOP of 19 - 24 mmHg, IYUZEH lowered IOP by 3 – 8 mmHg versus 4 – 8 mmHg by latanoprost ophthalmic solution preserved with benzalkonium chloride. Latanoprost ophthalmic solution preserved with benzalkonium chloride was approximately 1 mmHg more effective than IYUZEH.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
IYUZEH (latanoprost ophthalmic solution) is an opalescent, isotonic, white to slightly yellow solution of latanoprost 50 mcg/mL (0.005%) practically free from foreign particles.
It is supplied as a sterile solution in translucent low-density polyethylene single-dose container packaged in foil pouches (5 single-dose containers per pouch).
NDC 82584-003-30; Unit-of-Use Carton of 30
Storage:
Store at 15°C to 25°C (59°F to 77°F).
Store in the original pouch. After the pouch is opened, the single-dose containers may be stored in the opened foil pouch for up to 30 days at room temperature 15°C to 25°C (59°F to 77°F).
Patient should be advised to write down the date the foil pouch is opened in the space provided on the pouch.
Discard any unused containers 30 days after first opening the pouch.
-
17 PATIENT COUNSELING INFORMATION
Potential for Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Inform patients about the possibility of eyelid skin darkening, which may be reversible after discontinuation of IYUZEH.
Potential for Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with latanoprost ophthalmic solution. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Advise patients that IYUZEH is a sterile solution that does not contain a preservative. The drops are supplied in single-dose container. The solution from one individual container is to be used immediately after opening for administration to one or both eyes. Since sterility cannot be maintained after the individual container is opened, the remaining contents should be discarded immediately after administration. Open a new single-dose container every time you use IYUZEH.
When to Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of IYUZEH.
Contact Lens Use
Advise patients that contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes following administration of IYUZEH.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart.
If a Dose is Missed
Advise patients that if one dose is missed, treatment should continue with the next dose as normal.
Manufactured for: Thea Pharma Inc. Waltham, MA 02451.
All rights reserved.
U.S. Patent N°. 8,637,054. ©2021, Laboratoires Théa. All rights reserved. IYUZEH ™ is a trademark of Laboratoires Théa.
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 12/2022 PATIENT INFORMATION
IYUZEH ™ (eye yoo’ zeh)
(latanoprost ophthalmic solution) 0.005%
For topical ophthalmic useWhat is IYUZEH?
IYUZEH is a prescription sterile eye drop solution which does not contain a preservative. IYUZEH is used to lower the pressure in the eye (intraocular pressure) in people with open-angle glaucoma or ocular hypertension when their eye pressure is too high. IYUZEH belongs to a group of medicines called prostaglandin analogs.
It is not known if IYUZEH is safe and effective in children.Do not use IYUZEH if you are allergic to latanoprost or any of the ingredients in IYUZEH. See the end of this Patient Information leaflet for a complete list of ingredients in IYUZEH.
Before you use IYUZEH, tell your doctor if you:
- have or have had eye problems including any surgery on your eye or eyes
- are using any other eye medicines
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if IYUZEH will harm your unborn baby. If you become pregnant while using IYUZEH talk to your doctor right away.
- are breastfeeding or plan to breastfeed. It is not known if IYUZEH passes into your breast milk. Talk to your doctor about the best way to feed your baby if you use IYUZEH.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take IYUZEH?
Read the Instructions for Use at the end of this Patient Information leaflet for additional instructions about the right way to use IYUZEH.
- Use 1 drop of IYUZEH in your eye (or eyes) each evening. Talk to your doctor or pharmacist if you are not sure how to use IYUZEH.
- Your IYUZEH may not work as well if you use it more than 1 time each evening.
- If you miss a dose of IYUZEH, skip the missed dose and take the next dose at your regular time.
- If you use other medicines in your eye, wait at least 5 minutes between using IYUZEH and your other eye medicines.
- Contact lenses should be taken out before using IYUZEH and you should wait at least 15 minutes after giving the dose of IYUZEH before putting the contact lenses back into your eyes.
- Use your IYUZEH right away after opening. Each IYUZEH single-dose container is sterile and is to be used 1 time then thrown away. Do not save any IYUZEH that may be left over after you use your medicine. Using IYUZEH that is not sterile may cause other eye problems.
What are the possible side effects of IYUZEH?
IYUZEH may cause serious side effects including:
- changes in the color of your eye (iris). Your iris may become more brown in color while using IYUZEH. This color change may not go away when you stop using IYUZEH. If IYUZEH is used in 1 eye only, the color of that eye may always be a different color from the color of your other eye.
- darkening of the color of the skin around your eye (eyelid). These skin changes usually go away when you stop using IYUZEH.
- increasing the length, thickness, color, or number of your eyelashes. These eyelash changes usually go away when you stop using IYUZEH.
- hair growth on your eyelids. This hair growth usually goes away when you stop using IYUZEH.
The most common side effects of IYUZEH include:
- redness of and around the eye (conjunctival hyperemia)
- eye irritation
- eye itching
- abnormal sensation in the eye
- foreign body sensation in the eye
- blurry vision
- increase of tears in the eye (increased lacrimation)
Tell your doctor right away if you have any new eye problems while using IYUZEH, including:
- an eye injury
- an eye infection
- a sudden loss of vision
- eye surgery
- swelling and redness of and around your eye (conjunctivitis)
- problems with your eyelids
Additionally, the following side effects have been reported in other latanoprost medicines like IYUZEH, when used in the eye (topical use):
- dizziness
- headache
- peeling skin over much of the body (toxic epidermal necrolysis)
- worsening of asthma
- shortness of breath (dyspnea)
- itching
- viral infection of the cornea (herpes keratitis)
- chest pain caused by interruptions in the heart’s blood supply, that can occur at rest (angina, unstable angina)
- awareness of heart rhythm (palpitations)
- chest pain
Tell your doctor if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of IYUZEH. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store IYUZEH?
Before opening the foil pouches:
- Store the unopened foil pouches between 59°F to 77°F (15°C to 25°C).
- Do not open the foil pouch containing IYUZEH until you are ready to use the eye drops.
After opening the foil pouch:
- Store the opened foil pouch between 59°F to 77°F (15°C to 25°C), for up to 30 days.
- Write down the date of first opening the foil pouch in the space provided on the pouch.
- Throw away all unused IYUZEH single-dose containers in the opened foil pouch after 30 days.
- Keep the IYUZEH single-dose containers in their original foil pouch.
Keep IYUZEH and all medicines out of the reach of children.
General information about the safe and effective use of IYUZEH.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use IYUZEH for a condition for which it was not prescribed. Do not give IYUZEH to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or doctor for information about IYUZEH that is written for health professionals.
What are the ingredients in IYUZEH?
Active ingredients: latanoprost
Inactive ingredients: polyoxyl 40 hydrogenated castor oil, sorbitol, carbomer 974P, polyethylene glycol 4000, disodium edetate, sodium hydroxide (for pH-adjustment) and water.
Manufactured for: Thea Pharma Inc. Waltham, MA 02451.
All rights reserved.
U.S. Patent N°. 8,637,054. ©2021, Laboratoires Théa. All rights reserved. IYUZEH™ is a trademark of Laboratoires Théa.
-
INSTRUCTIONS FOR USE
Instructions for Use Read these Instructions for Use before using your IYUZEH™ and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
Important:
- IYUZEH is for the eye. Do not swallow IYUZEH.
- IYUZEH single-dose containers are packaged in a foil pouch.
- Do not dose the IYUZEH single-dose containers if the foil pouch is opened.
- Write down the date you open the foil pouch in the space provided on the pouch.
- Do not open the IYUZEH single-dose container until you are ready to use the eye drops.
Please follow these instructions to use IYUZEH:
Step 1.
Wash your hands and sit or stand comfortably.
Step 2.
Open the foil pouch containing a strip of 5 single-dose containers.
Write down the date of first opening on the foil pouch.
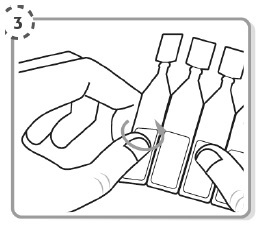
Step 3.
Take the strip of single-dose containers from the foil pouch
Break off one single-dose container from the strip
Place the unopened single-dose containers back in the foil pouch and fold the edge to close the pouch.
Step 4.
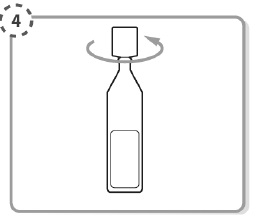
Hold the single-dose container upright.
Make sure that your IYUZEH medicine is in the bottom part of the single-dose container.
Twist open the top of the single-dose container as shown. Do not touch the tip after opening the container.
Step 5.
Tilt your head backwards. If you are unable to tilt your head, lie down.
Use your finger to gently pull down the lower eyelid of your affected eye.
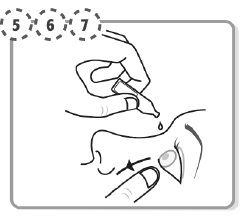
Step 6.
Place the tip of the single-dose container close to, but not touching your eye.
Step 7.
Squeeze the single-dose container gently so that only one drop goes into your eye, then release the lower eyelid.
If the drop misses your eye completely, try again.- If your doctor has told you to use IYUZEH drops in both eyes, repeat Steps 5 to Step 7 for your other eye.
- Each single-dose container contains enough solution for both eyes.
- Throw away the single-dose container after use. Do not keep it to use it again. To lessen the chance of an infection, a new single-dose container must be opened each time you are ready to use IYUZEH.
- Place the unopened foil pouch with the unopened single-dose containers back in the carton. The unopened single-dose containers must be used within 30 days after opening the foil pouch.
Rx

Manufactured for: Thea Pharma Inc. Waltham, MA 02451.
All rights reserved.
U.S. Patent N°. 8,637,054. ©2021, Laboratoires Théa. All rights reserved. IYUZEH™ is a trademark of Laboratoires Théa.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Approved: 12/2022
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IYUZEH
latanoprost ophthalmic solution 0.005% solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82584-003 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LATANOPROST (UNII: 6Z5B6HVF6O) (LATANOPROST ACID - UNII:EJ85341990) LATANOPROST 50 ug in 1 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82584-003-30 30 in 1 CARTON 07/14/2023 1 0.2 mL in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216472 07/14/2023 Labeler - Thea Pharma Inc. (117787029)