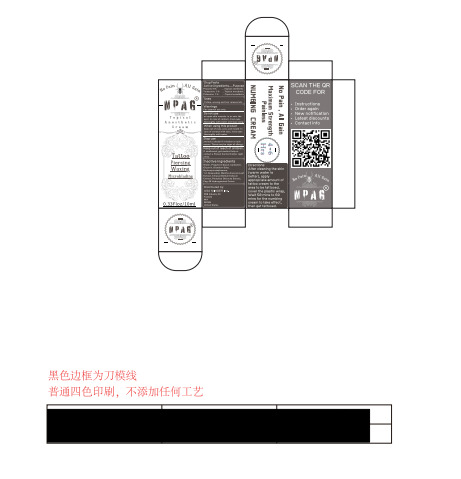
Label: NPAG TATTOO NUMBING CREAM cream
- NDC Code(s): 84353-001-01
- Packager: USA MINGER Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
NPAG TATTOO NUMBING CREAM
npag tattoo numbing cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84353-001 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROCAINE (UNII: 4Z8Y51M438) (PROCAINE - UNII:4Z8Y51M438) PROCAINE 5 g in 100 mL TETRACAINE (UNII: 0619F35CGV) (TETRACAINE - UNII:0619F35CGV) TETRACAINE 1 g in 100 mL PRILOCAINE HYDROCHLORIDE (UNII: MJW015BAPH) (PRILOCAINE - UNII:046O35D44R) PRILOCAINE HYDROCHLORIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84353-001-01 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/24/2024 Labeler - USA MINGER Inc. (123952552) Establishment Name Address ID/FEI Business Operations Guangdong Aimu Biological Technology Co., Ltd 712647107 manufacture(84353-001) Establishment Name Address ID/FEI Business Operations USA MINGER Inc 123952552 label(84353-001)