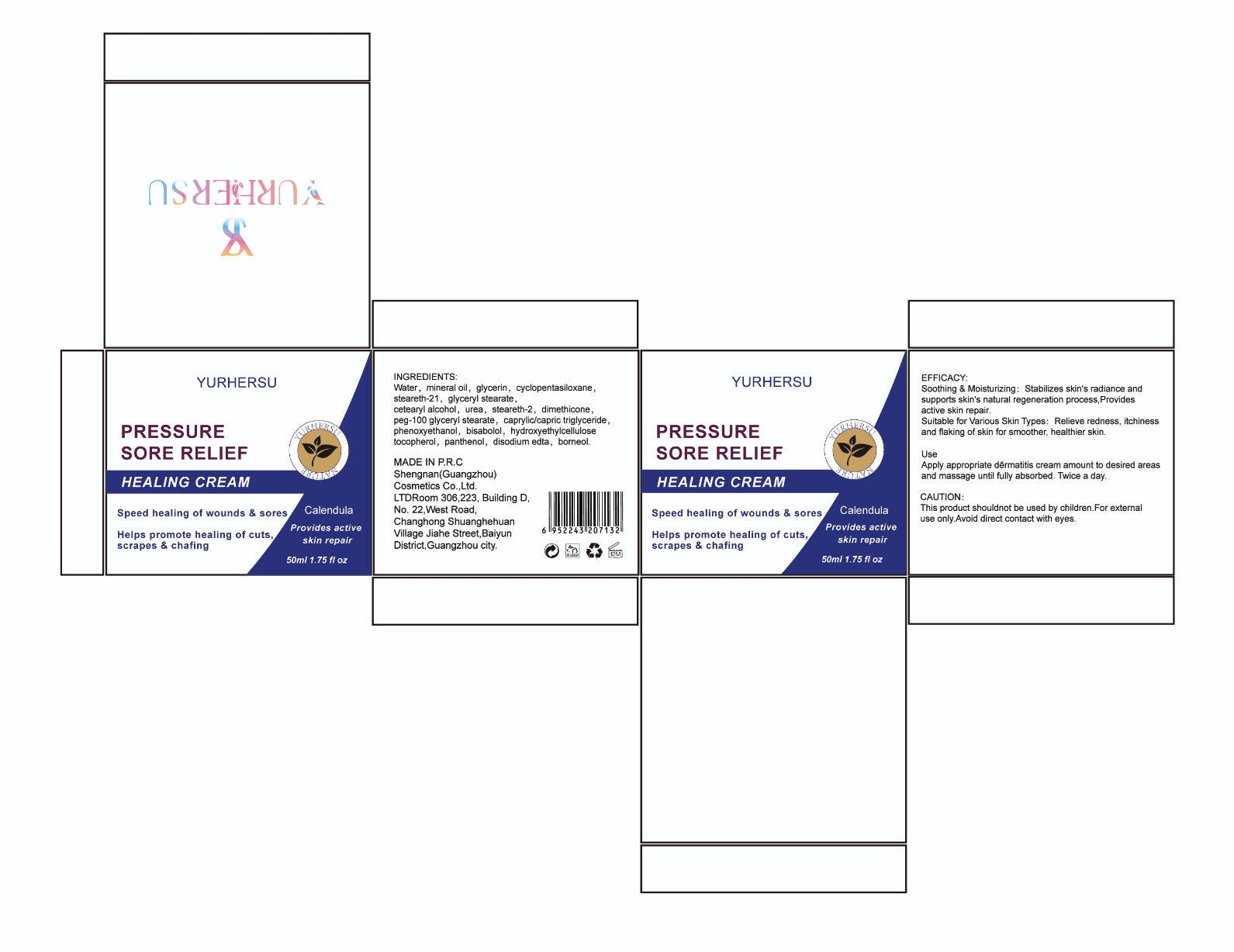
Label: HEALING CREAM- borneol cream
- NDC Code(s): 84019-002-01
- Packager: Shengnan (Guangzhou) Cosmetics Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Warnings
- Stop use and ask a doctor if
- Do not use
- When using this product
- Keep out of reach of children.
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEALING CREAM
borneol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84019-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BORNEOL (UNII: M89NIB437X) (BORNEOL - UNII:M89NIB437X) BORNEOL 2 g in 100 mL Inactive Ingredients Ingredient Name Strength DODECANEDIOIC ACID (UNII: 978YU42Q6I) CETYL HYDROXYETHYLCELLULOSE (350000 MW) (UNII: T7SWE4S2TT) TOCOPHEROL (UNII: R0ZB2556P8) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) .BETA.-BISABOLOL (UNII: LP618AV2EA) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MINERAL OIL (UNII: T5L8T28FGP) PANTHENOL (UNII: WV9CM0O67Z) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) CAPRYLIC ACID (UNII: OBL58JN025) WATER (UNII: 059QF0KO0R) (4-(TRIFLUOROMETHOXY)PHENYL)UREA (UNII: SQP4E8O29W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84019-002-01 1 in 1 BOX 05/23/2024 1 50 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/23/2024 Labeler - Shengnan (Guangzhou) Cosmetics Co., LTD (541200425) Establishment Name Address ID/FEI Business Operations Shengnan (Guangzhou) Cosmetics Co., LTD 541200425 manufacture(84019-002)