Label: MEDACTIVE ORAL RELIEF- spray spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 49741-1019-1 - Packager: Integrate Oral Care, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 30, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Sore Throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly. If sore mouth symptoms do not improve in 7 days, see your dentist or doctor promptly.
If an excessive amount of spray is accidentally swwallowed, get medical help or contact a Poison Control Center right away. Avoid contact with eyes.
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Use as needed or as directed by a dentist or doctor.
- Adults and children 2 years or age and older: Hold bottle upright. Gently press down on spray and apply directly to affected areas in the mouth, approximately 3-4 times per application. Do not rinse out.
- Children under 12 years of age: Should be supervised in the use of this product.
- Children under 2 years of age: Consult a dentist or doctor.
-
OTHER SAFETY INFORMATION
Other Information
- Store at room temperature. Safe to swallow.
- Do not use if safety sleeve or label is damaged prior to first use. Do not push down on spray rapidly or excessively, as it may cause blockage.
- You may experience a slight, temporary tingle sensation upon initial use; this is normal and cuases no harm.
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MEDACTIVE ORAL RELIEF
spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49741-1019 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 29.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1 mg in 1 mL XYLITOL (UNII: VCQ006KQ1E) 1 mg in 1 mL DIMETHICONE (UNII: 92RU3N3Y1O) 1 mg in 1 mL EDETIC ACID (UNII: 9G34HU7RV0) 1 mg in 1 mL POLOXAMER 407 (UNII: TUF2IVW3M2) 1 mg in 1 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 1 mg in 1 mL SUCRALOSE (UNII: 96K6UQ3ZD4) 1 mg in 1 mL SACCHARIN SODIUM (UNII: SB8ZUX40TY) 1 mg in 1 mL XANTHAN GUM (UNII: TTV12P4NEE) 1 mCi in 1 mL Product Characteristics Color Score Shape Size Flavor VANILLA (Vanillamint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49741-1019-1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 08/31/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 08/31/2015 Labeler - Integrate Oral Care, LLC. (063540457) Registrant - Integrate Oral Care, LLC. (063540457)

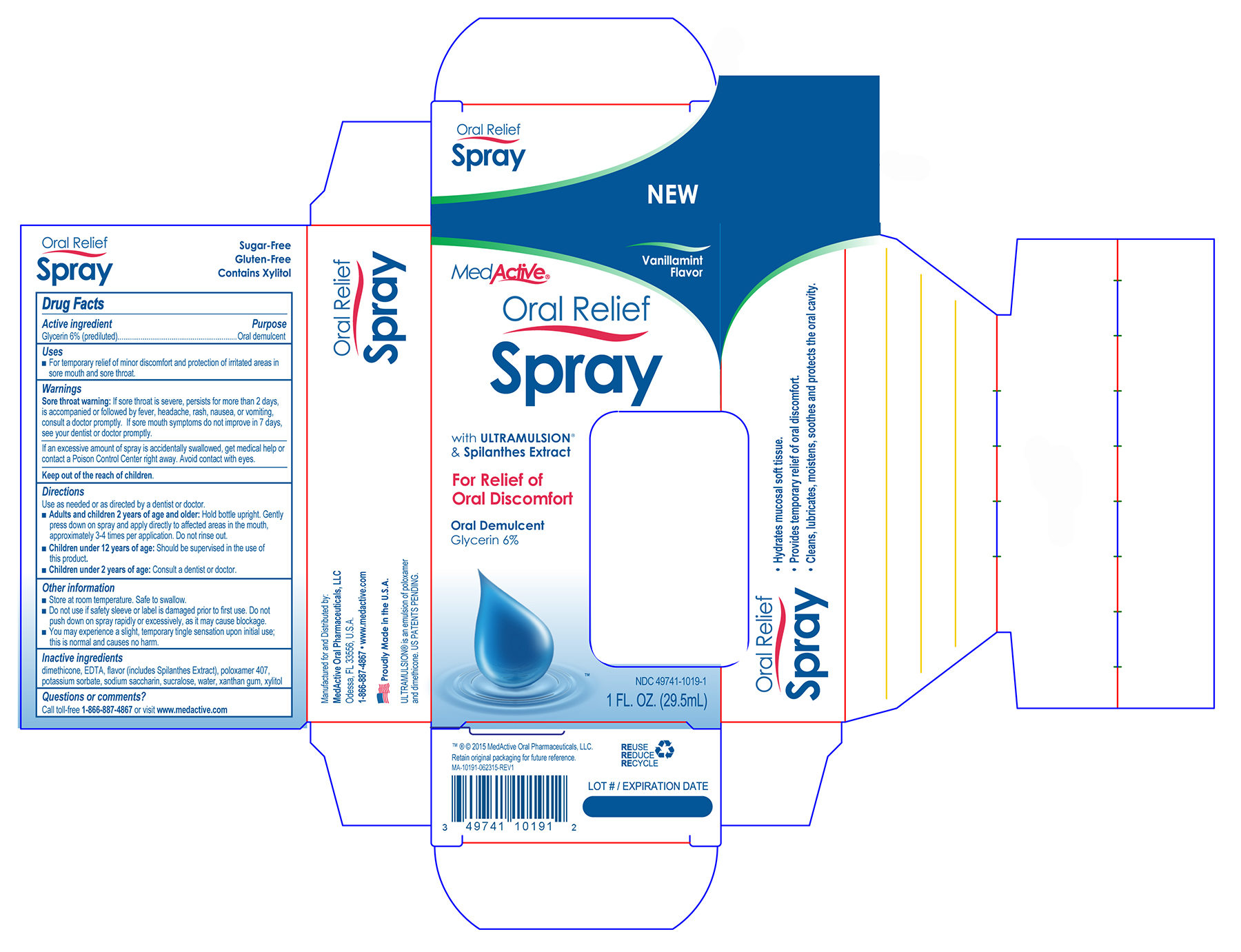
 NEW Vanillamint Flavor
NEW Vanillamint Flavor