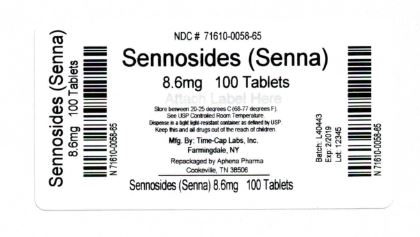
Label: SENEXON- senna tablet, coated
- NDC Code(s): 71610-058-65
- Packager: Aphena Pharma Solutions - Tennessee, LLC
- This is a repackaged label.
- Source NDC Code(s): 0536-5904
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- ASK DOCTOR/PHARMACIST
- ASK DOCTOR
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Dosage and Administration
Adults and children 12 years and over - 2 tablets once a day - maximum dosage - 4 tablets twice a day
Children 6 to under 12 years - 1 tablet once a day maximum dosage - 2 tablets twice a day
children 2 to under 6 years - 1/2 tablet once a day - maximum dosage - 1 tablet twice a day
- INDICATIONS & USAGE
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count 8.6 mg 100 71610-058-65 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20180514JH - PRINCIPAL DISPLAY PANEL - 8.6 mg
-
INGREDIENTS AND APPEARANCE
SENEXON
senna tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71610-058(NDC:0536-5904) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A and B (UNII: 1B5FPI42EN) (SENNOSIDES A and B - UNII:1B5FPI42EN) SENNOSIDES A and B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) mineral oil (UNII: T5L8T28FGP) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code TCL080 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71610-058-65 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 02/09/2006 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 REPACK(71610-058)