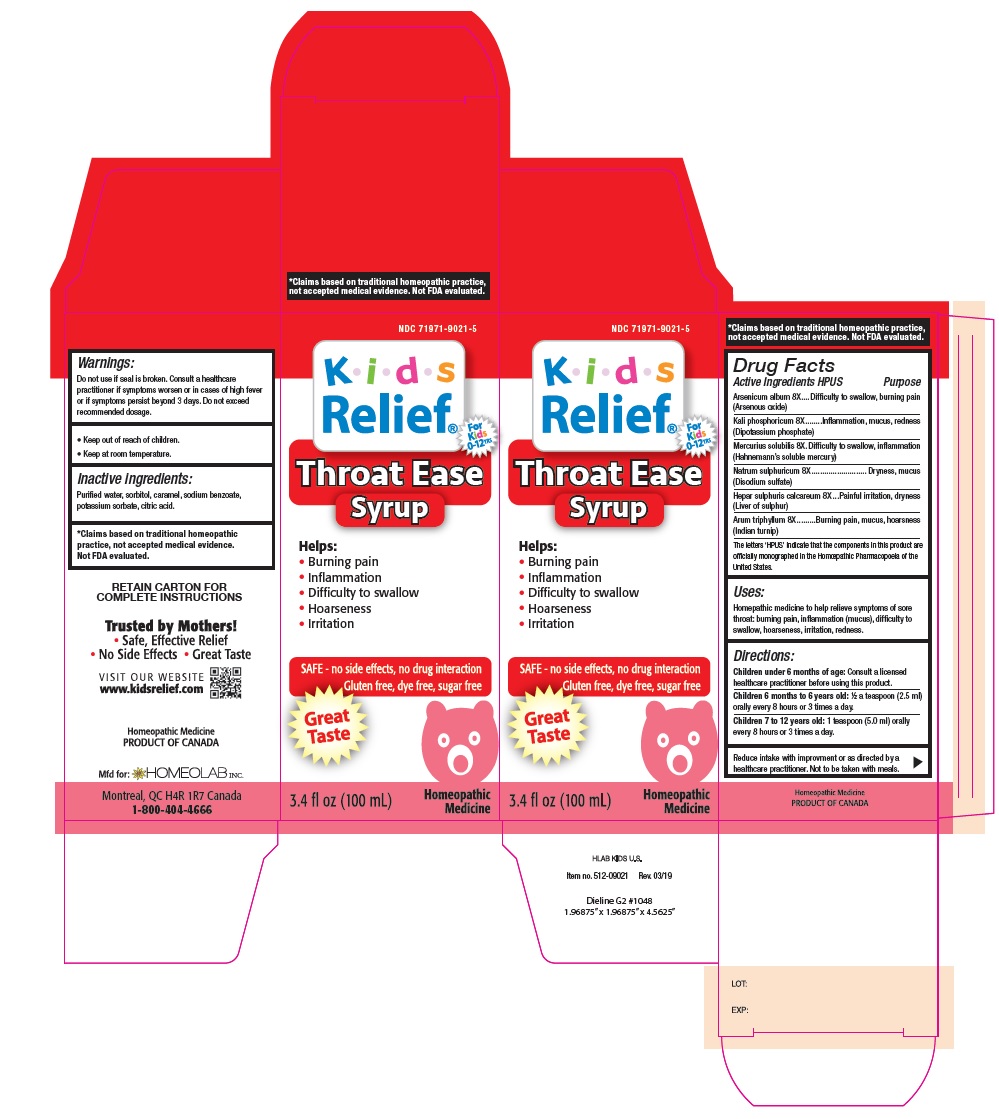
Label: KIDS RELIEF- arsenicum album, kali phosphoricum, mercuris solubilis, natrum sulphuricum, hepar sulphuris calcareum, arum triphyllum liquid
- NDC Code(s): 71971-9021-4, 71971-9021-5, 71971-9021-9
- Packager: Homeolab International (Canada) inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 23, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
PURPOSE
Purpose :
Homeopathic remedy helps relieve symptoms of sore throat, burning pain, inflammation (mucus), difficulty to swallow, hoarseness and irritation.
The letters 'HPUS' indicate that the components in this product are officially monographed in the Homoeopathic Pharmacopoeia of the United States.
*Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated - INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
Children under 6 months of age: Consult a licensed healthcare practitioner before using this product.
Children 6 months to 6 years old: ½ a teaspoon (2.5 ml) orally every 8 hours or 3 times a day.
Children 7 to 12 years old: 1 teaspoon (5.0 ml) orally every 8 hours or 3 times a day.
Reduce intake with improvements.
Not to be taken with meals - KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- Principal display panel
-
INGREDIENTS AND APPEARANCE
KIDS RELIEF
arsenicum album, kali phosphoricum, mercuris solubilis, natrum sulphuricum, hepar sulphuris calcareum, arum triphyllum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71971-9021 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 8 [hp_X] in 100 mL DIBASIC POTASSIUM PHOSPHATE (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) DIBASIC POTASSIUM PHOSPHATE 8 [hp_X] in 100 mL MERCURIUS SOLUBILIS (UNII: 324Y4038G2) (MERCURIUS SOLUBILIS - UNII:324Y4038G2) MERCURIUS SOLUBILIS 8 [hp_X] in 100 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 8 [hp_X] in 100 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 8 [hp_X] in 100 mL ARISAEMA TRIPHYLLUM ROOT (UNII: DM64K844DM) (ARISAEMA TRIPHYLLUM ROOT - UNII:DM64K844DM) ARISAEMA TRIPHYLLUM ROOT 8 [hp_X] in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CARAMEL (UNII: T9D99G2B1R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71971-9021-4 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2019 2 NDC:71971-9021-5 1 in 1 CARTON 08/01/2019 2 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:71971-9021-9 1 in 1 CARTON 08/01/2019 3 250 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/01/2019 Labeler - Homeolab International (Canada) inc (203639455)