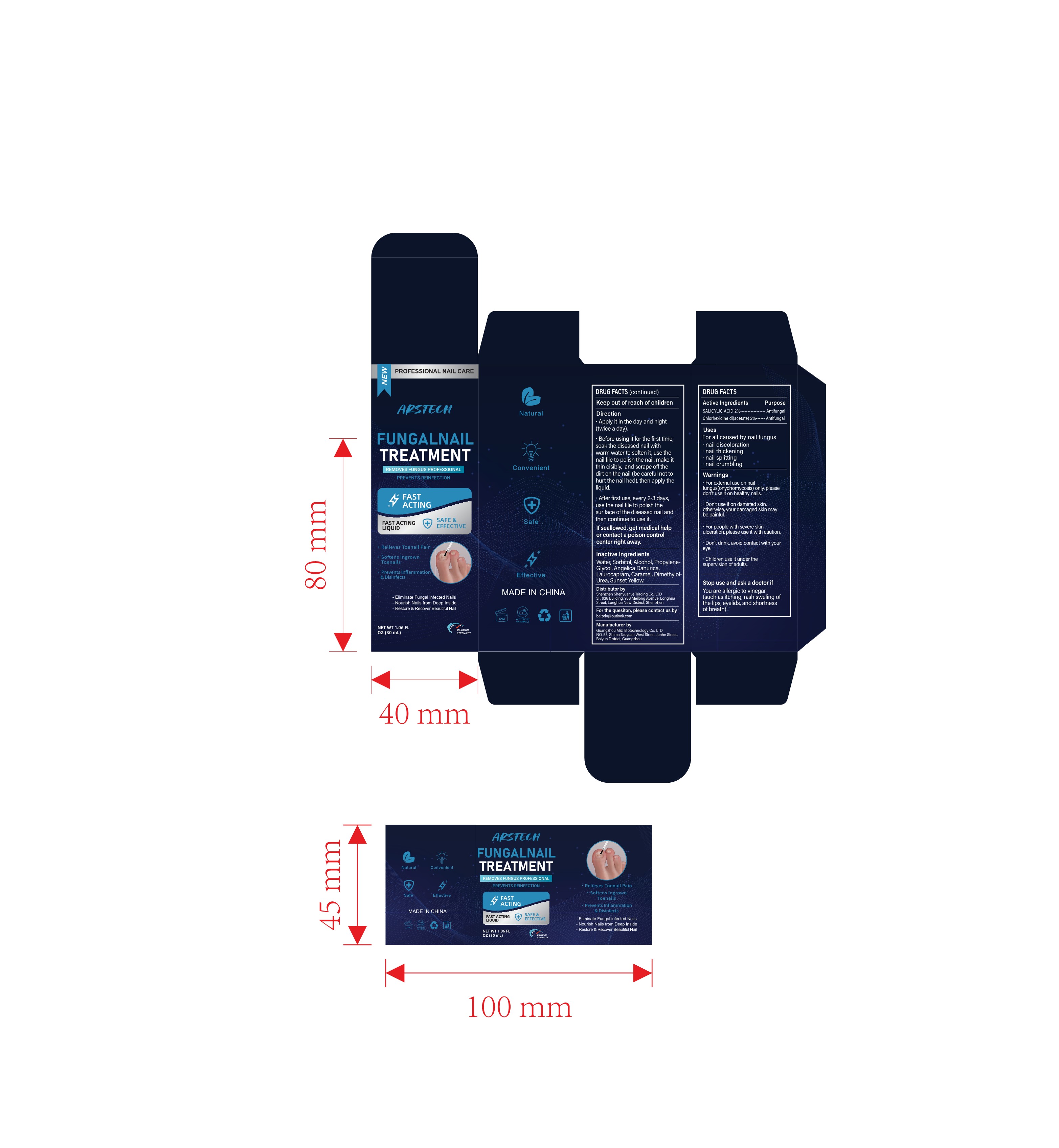
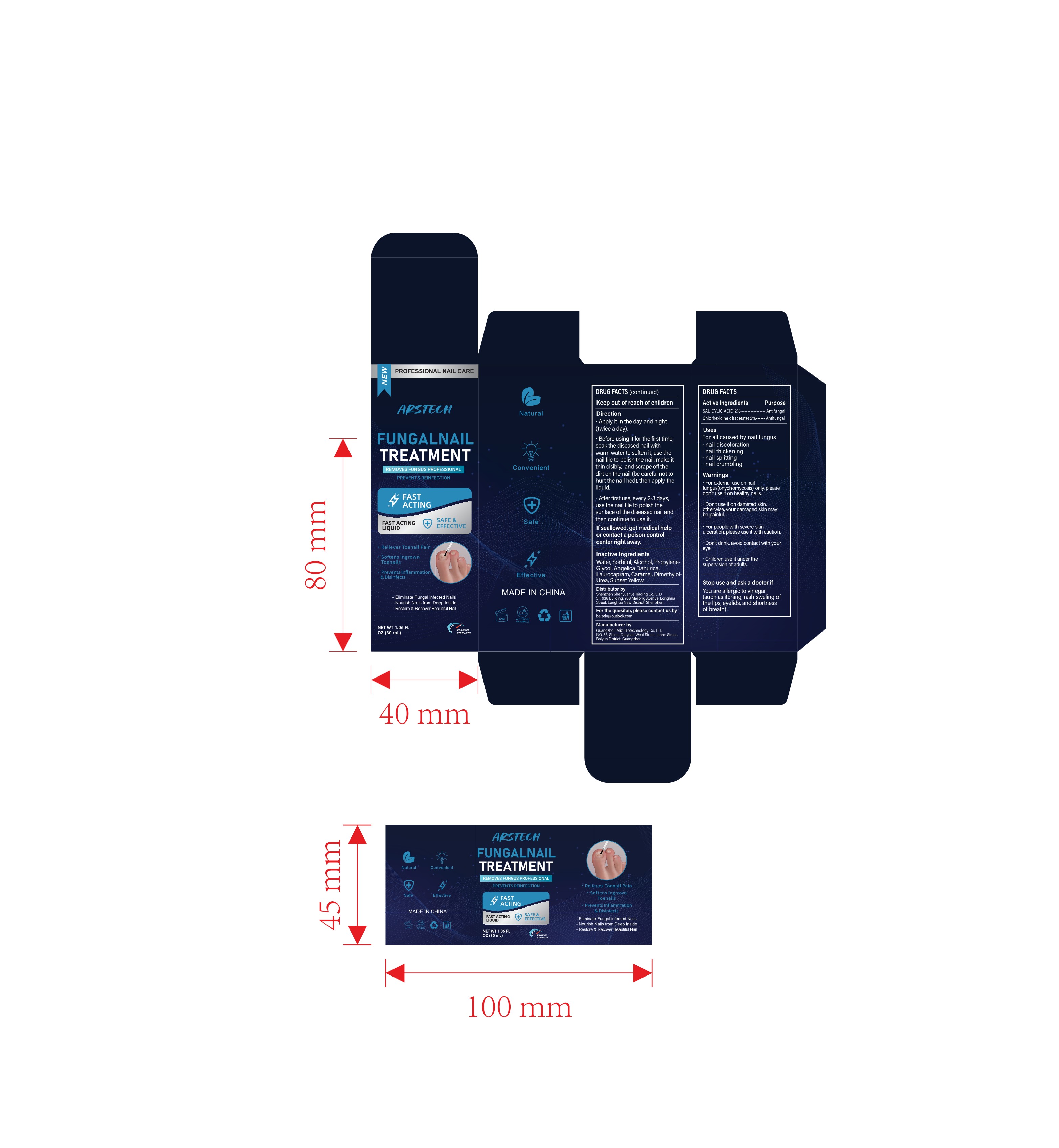
Label: NAIL TREATMENT LIQUID (salicylic acid, chlorhexidine di- acetate liquid
- NDC Code(s): 84336-002-01
- Packager: Shenzhen Shenyuanye Trading Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- ASK DOCTOR
-
DOSAGE & ADMINISTRATION
Direction
Apply it in the day and night(twice a day).
Before using it for the first time,soak the diseased nail with warm water to soften it, use thenail file to polish the nail, make itthin cisibly, and scrape off thedirt on the nail (be careful not tohurt the nail hed), then apply theliquid.
After first use, every 2-3 daysuse the nail file to polish thesur face of the diseased nail and then continue to use it.
If seallowed, get medical help or contact a poison controlcenter right away. - INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NAIL TREATMENT LIQUID
salicylic acid, chlorhexidine di(acetate) liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84336-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE ACETATE (UNII: 5908ZUF22Y) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE 2 mg in 100 mL SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) LAUROCAPRAM (UNII: 1F3X9DRV9X) CARAMEL (UNII: T9D99G2B1R) WATER (UNII: 059QF0KO0R) OXYMETHUREA (UNII: N68H97CAWG) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84336-002-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/11/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 06/11/2024 Labeler - Shenzhen Shenyuanye Trading Co., LTD (550376513) Registrant - Shenzhen Shenyuanye Trading Co., LTD (550376513) Establishment Name Address ID/FEI Business Operations Guangzhou Mizi Biotechnology Co., Ltd. 418001649 manufacture(84336-002)