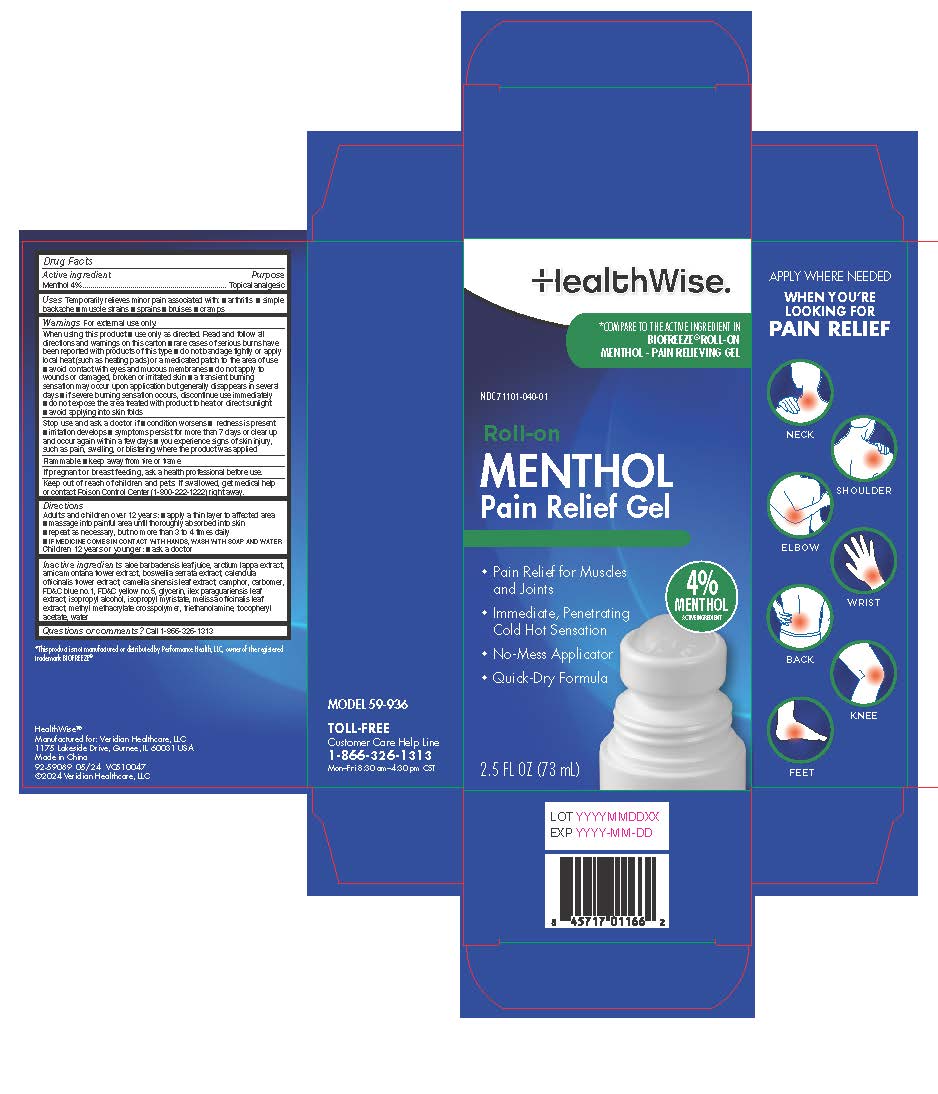
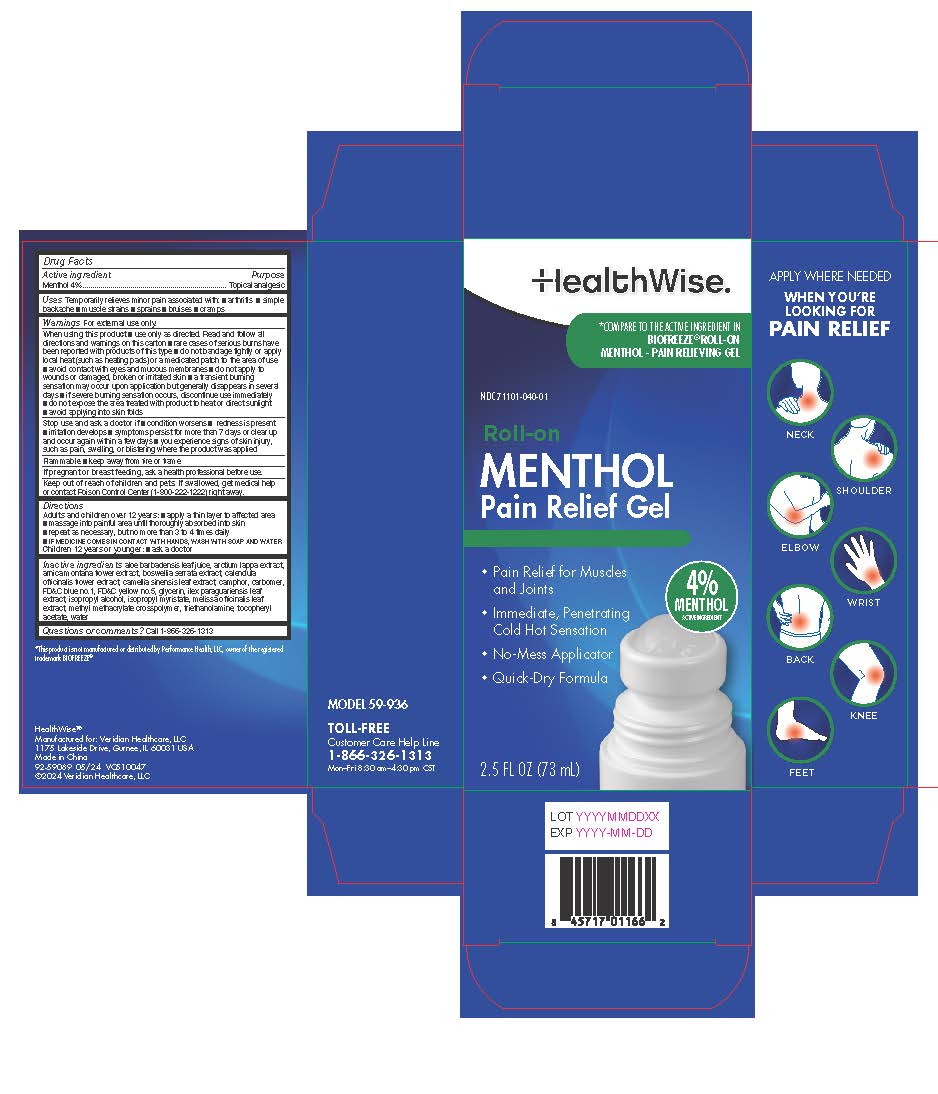
Label: HEALTHWISE MENTHOL 4% ROLL-ON- menthol liquid
- NDC Code(s): 71101-040-01
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
When using this product
■ use only as directed. Read and follow all directions and warnings on this carton
■ rare cases of serious burns have been reported with products of this type
■ do not bandage tightly or apply local heat (such as heating pads) or a medicated patch to the area of use
■ avoid contact with eyes and mucous membranes■ do not apply to wounds or damaged, broken or irritated skin
■ a transient burning sensation may occur upon application but generally disappears in several days
■ if severe burning sensation occurs, discontinue use immediately
■ do not expose the area treated with the product to heat or direct sunlight
■ avoid applying into skin foldsStop use and ask a doctor if
- condition worsens
- redness is present
- irritation develops
- symptoms persist for more than 7 days or clear up and occur again within a few days
- you experience signs of skin injury, such as pain, swelling, or blistering where the product was applied
Flammable■ Keep away from fire or flame
-
Directions
Adults and children over 12 years: ■ apply a thin layer to affected area
■ massage into painful area until thoroughly absorbed into skin
■ repeat as necessary, but no more than 3 to 4 times daily
■ IF MEDICINE COMES IN CONTACT WITH HANDS, WASH WITH SOAP AND WATER
Children 12 years or younger: ■ ask a doctor -
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer (6000 MPA.S), capsaicin, glycerin, isopropyl myristate, propylene glycol, SD alcohol, triethanolamine, water
Inactive ingredients acrylates/C10-30 alkyl acrylate crosspolymer (60000 MPA.S), aloe barbadensis leaf juice, aminomethyl propanol, ceteth-10 phosphate, cetostearyl alcohol, Cyclomethicone 5, dicetyl phosphate, dimethicone 350, dimethicone/vinyl dimethicone
crosspolymer (soft particle), disodium EDTA, ethylhexylglycerin, glyceryl 1 stearate, hydroxyethyl acrylate/sodium acryloyl dimethyl taurate copolymer (100000 MPA.S at 1.5%), isohexadecane,
phenoxyethanol, SD alcohol, steareth-21, water
- Questions or comments
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HEALTHWISE MENTHOL 4% ROLL-ON
menthol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 4 g in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER 940 (UNII: 4Q93RCW27E) CAMPHOR (NATURAL) (UNII: N20HL7Q941) TROLAMINE (UNII: 9O3K93S3TK) ALCOHOL (UNII: 3K9958V90M) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) WATER (UNII: 059QF0KO0R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) ARCTIUM LAPPA WHOLE (UNII: 73070DU1LA) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-040-01 1 in 1 CARTON 05/01/2024 1 73 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/01/2024 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc 421280161 manufacture(71101-040)