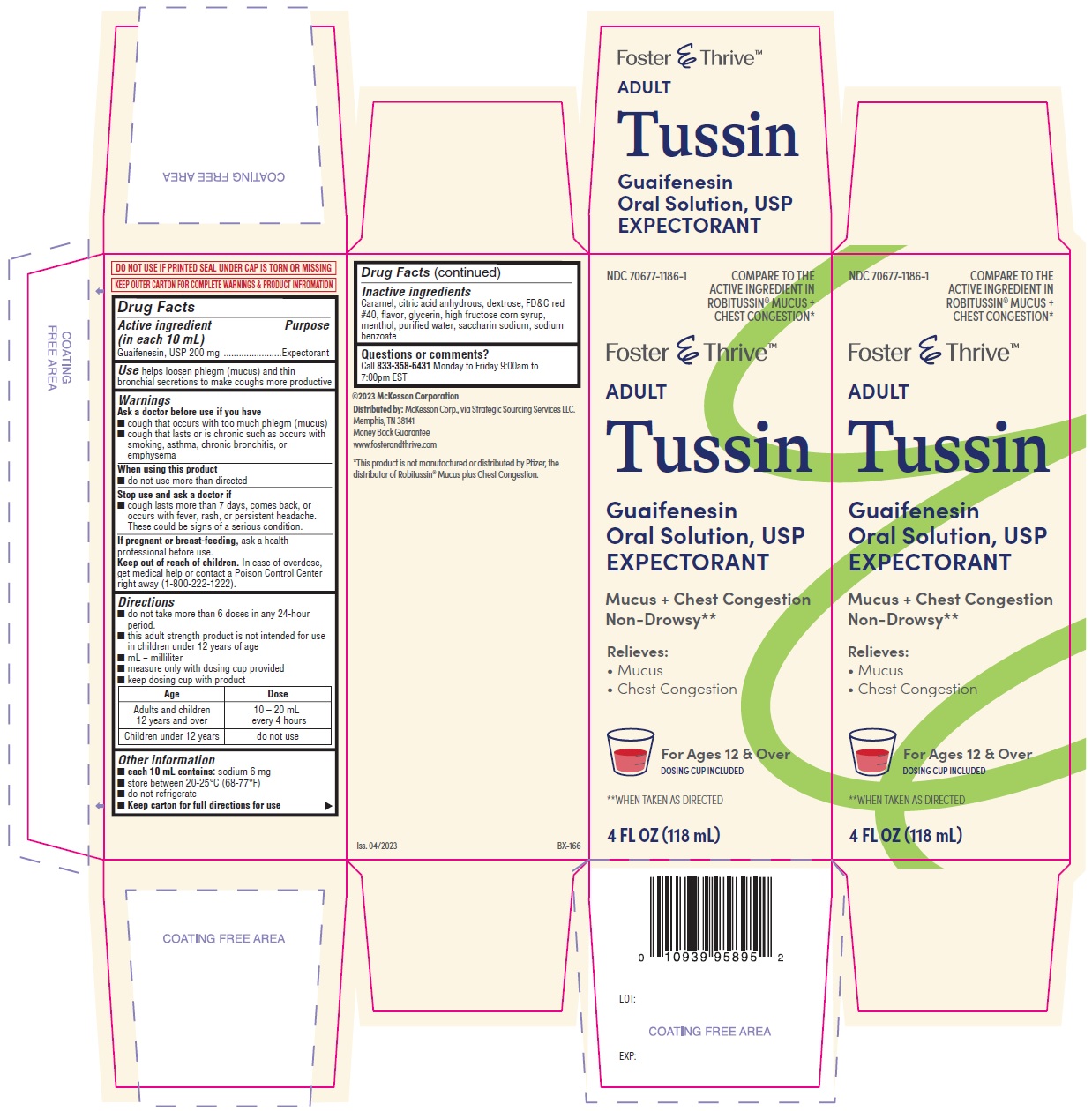
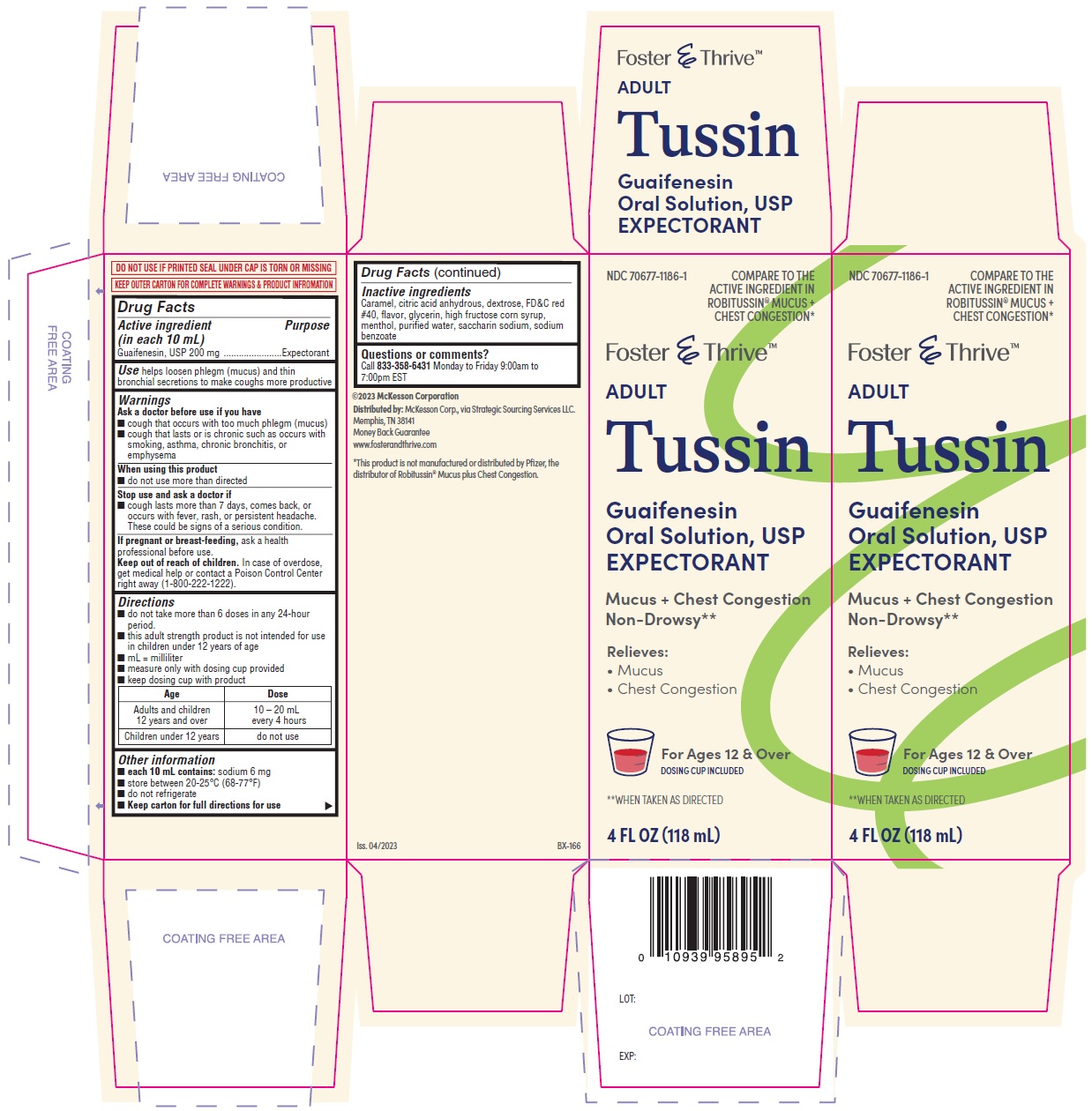
Label: TUSSIN- guaifenesin solution
- NDC Code(s): 70677-1186-1, 70677-1186-2
- Packager: Strategic Sourcing Services
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- If pregnant or breast-feeding
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
■ do not take more than 6 doses in any 24-hour period.
■ this adult strength product is not intended for use in children under 12 years of age
■ mL = milliliter
■ measure only with dosing cup provided
■ keep dosing cup with product
Age
Dose
Adults and children
12 years and over
10 – 20 mL
every 4 hours
Children under 12 years
do not use
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TUSSIN
guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70677-1186 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL Inactive Ingredients Ingredient Name Strength CARAMEL (UNII: T9D99G2B1R) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) DEXTROSE (UNII: IY9XDZ35W2) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) MENTHOL (UNII: L7T10EIP3A) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70677-1186-1 1 in 1 CARTON 08/09/2023 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:70677-1186-2 1 in 1 CARTON 08/09/2023 2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/09/2023 Labeler - Strategic Sourcing Services (116956644) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(70677-1186)