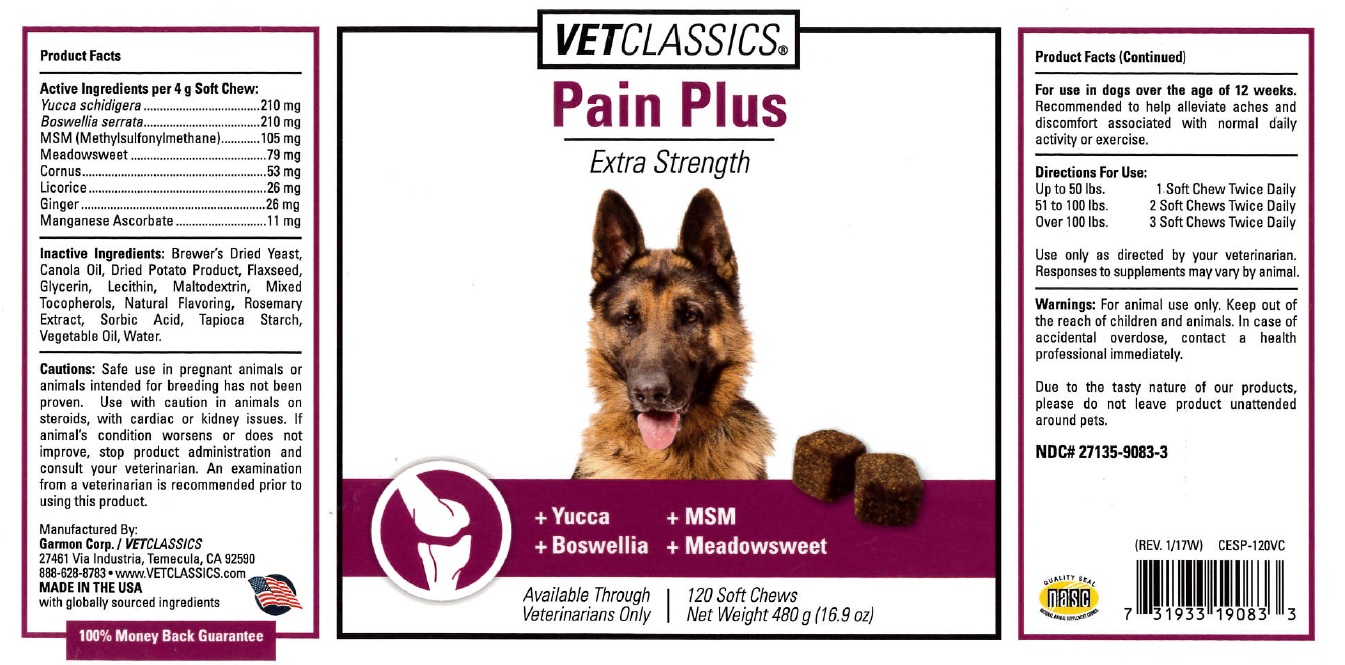
Label: CESP - PAIN PLUS SOFT CHEW- pain plus soft chew pellet
- NDC Code(s): 27135-9083-3
- Packager: Garmon Corp
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 8, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Product Facts
- INACTIVE INGREDIENT
-
WARNINGS AND PRECAUTIONS
Cautions: Safe use in pregnant animals or animals intended for breeding has not been proven. Use with caution in animals on steriods, with cardiac or kidney issues. If animal's condition worsens or does not improve, stop product administration and consult your veterinarian. An examination from a veterinarian is recommended prior to using this product.
- Product Facts (Continued)
- DOSAGE & ADMINISTRATION
- WARNINGS AND PRECAUTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CESP - PAIN PLUS SOFT CHEW
pain plus soft chew pelletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:27135-9083 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength YUCCA SCHIDIGERA (UNII: 08A0YG3VIC) (YUCCA SCHIDIGERA - UNII:08A0YG3VIC) YUCCA SCHIDIGERA 210 mg in 4 g BOSWELLIA SERRATA WHOLE (UNII: X7B7P649WQ) (BOSWELLIA SERRATA WHOLE - UNII:X7B7P649WQ) BOSWELLIA SERRATA WHOLE 210 mg in 4 g DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) (DIMETHYL SULFONE - UNII:9H4PO4Z4FT) DIMETHYL SULFONE 105 mg in 4 g FILIPENDULA ULMARIA WHOLE (UNII: 3LH0M209LN) (FILIPENDULA ULMARIA WHOLE - UNII:3LH0M209LN) FILIPENDULA ULMARIA WHOLE 79 mg in 4 g CORNUS ALBA WHOLE (UNII: W6JR5J2XFE) (CORNUS ALBA WHOLE - UNII:W6JR5J2XFE) CORNUS ALBA WHOLE 53 mg in 4 g LICORICE (UNII: 61ZBX54883) (LICORICE - UNII:61ZBX54883) LICORICE 26 mg in 4 g GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 26 mg in 4 g MANGANESE ASCORBATE (UNII: 332B7O278V) (MANGANESE ASCORBATE - UNII:332B7O278V) MANGANESE ASCORBATE 11 mg in 4 g Inactive Ingredients Ingredient Name Strength YEAST, UNSPECIFIED (UNII: 3NY3SM6B8U) CANOLA OIL (UNII: 331KBJ17RK) POTATO (UNII: CFE1S8DYWD) FLAX SEED (UNII: 4110YT348C) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MALTODEXTRIN (UNII: 7CVR7L4A2D) TOCOPHEROL (UNII: R0ZB2556P8) CHICKEN (UNII: 0X8Q245Y7B) ROSEMARY (UNII: IJ67X351P9) SORBIC ACID (UNII: X045WJ989B) STARCH, TAPIOCA (UNII: 24SC3U704I) CORN OIL (UNII: 8470G57WFM) WATER (UNII: 059QF0KO0R) Product Characteristics Color brown Score Shape SQUARE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:27135-9083-3 480 g in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/08/2017 Labeler - Garmon Corp (011706236) Registrant - Garmon Corp (011706236) Establishment Name Address ID/FEI Business Operations Garmon Corp 011706236 manufacture, api manufacture