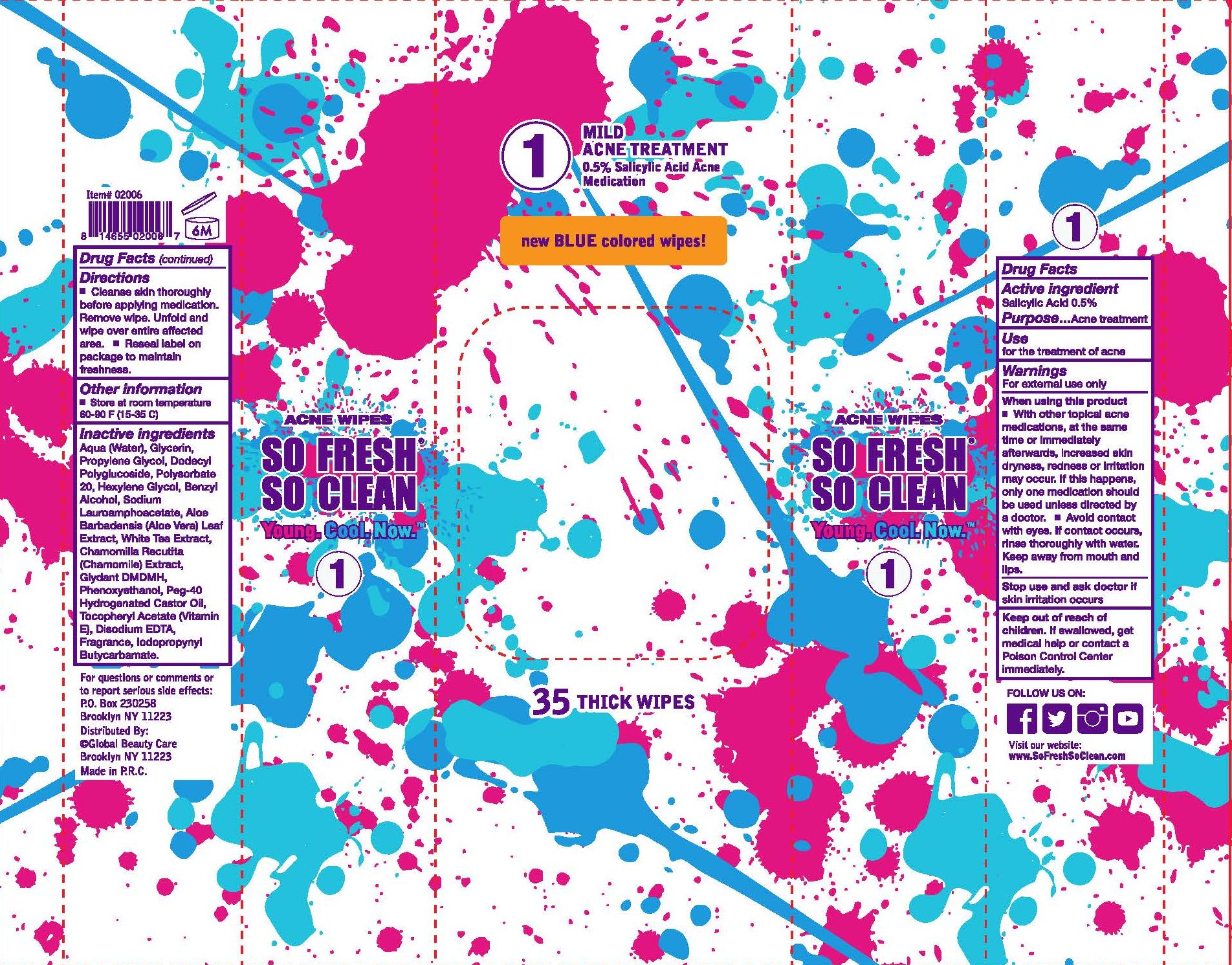
Label: SO FRESH SO CLEAN MILD ACNE TREATMENT- salicylic acid cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 69722-733-35 - Packager: Global Beauty Care, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 25, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
- SO FRESH SO CLEAN MILD ACNE TREATMENT 0.5% Salicylic Acid Acne Medication
- Active ingredient
- Purpose
- Use
- Warnings
-
When using this product
- With other topical acne medications, at the same time or immediately afterwards, increased skin dryness, redness or irritation may occur. If this happens, only one medication should be used unless directed by a doctor.
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water. Keep away from mouth and lips.
- Stop use and ask doctor if
- Keep out of reach of children.
- Directions
- Other information
-
Inactive ingredients
Aqua (Water), Glycerin, Propylene Glycol, Dodecyl Polyglucoside, Polysorbate 20, Hexylene Glycol, Benzyl Alcohol, Sodium Lauroamphoacetate, Aloe Barbadensis (Aloe Vera) Leaf Extract, White Tea Extract, Chamomilla Recutita (Chamomile) Extract, Glydant DMDMH, Phenoxyethanol, Peg-40 Hydrogenated Castor Oil, Tocopheryl Acetate (Vitamin E), Disodium EDTA, Fragrance, Iodopropynyl Butycarbamate.
- QUESTIONS
-
INGREDIENTS AND APPEARANCE
SO FRESH SO CLEAN MILD ACNE TREATMENT
salicylic acid clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69722-733 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 20 (UNII: 7T1F30V5YH) HEXYLENE GLYCOL (UNII: KEH0A3F75J) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM LAUROAMPHOACETATE (UNII: SLK428451L) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) EDETATE DISODIUM (UNII: 7FLD91C86K) ALOE VERA LEAF (UNII: ZY81Z83H0X) MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) DMDM HYDANTOIN (UNII: BYR0546TOW) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69722-733-35 35 in 1 BAG 06/25/2015 1 0.00135 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/25/2015 Labeler - Global Beauty Care, Inc. (068600947)