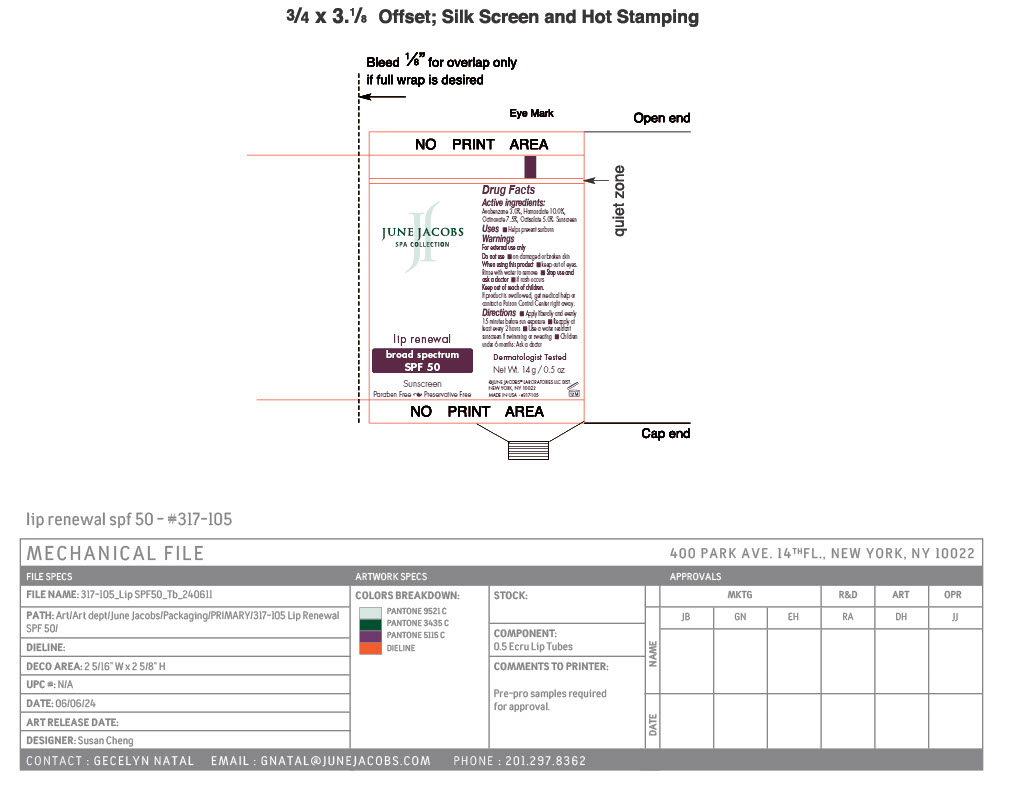
Label: LIP RENEWAL SPF 50- avobenzone, homosalate, octinoxate, octisalate paste
- NDC Code(s): 65278-105-01, 65278-105-02
- Packager: June Jacobs Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- Apply liberally and evenly every 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months: Ask a doctor
- Sun Protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use as sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun especially from 10am -2 pm.
- wear long sleeve shirt, pants hats and sunglasses
-
INACTIVE INGREDIENT
Inactive Ingredients
Microcrystalline Wax, Diisostearyl Malate, Glyceryl Diisostearate, Simmondsia Chinensis (Jojoba) Seed Oil, Copernicia Cerifera (Carnauba) Wax, Butyrospermum Parkii (Shea) Butter, Dimethicone, Glycerin, Vitis Vinifera (Grape) Seed Oil, Flavor (Aroma), Triisostearin, Tocopherol, Squalane, Lycium Barbarum Fruit Extract, Punica Granatum Extract, Vitis Vinifera (Grape) Seed Extract, Aspalathus Linearis Leaf Extract, Allantoin, Water/ Aqua, Leuconostoc/Radish Root Ferment Filtrate, Camellia Sinensis Leaf Extract, Hyaluronic Acid, Phenoxyethanol, Limonene, Citral
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIP RENEWAL SPF 50
avobenzone, homosalate, octinoxate, octisalate pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65278-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.05 g in 14 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.42 g in 14 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 1.4 g in 14 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.7 g in 14 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONIC ACID (UNII: S270N0TRQY) WATER (UNII: 059QF0KO0R) LIMONENE, (+)- (UNII: GFD7C86Q1W) MENTHOL (UNII: L7T10EIP3A) VANILLIN (UNII: CHI530446X) LYCIUM BARBARUM FRUIT (UNII: 930626MWDL) GLYCERYL DIISOSTEARATE (UNII: 68BAV42LRC) DIMETHICONE (UNII: 92RU3N3Y1O) GRAPE SEED OIL (UNII: 930MLC8XGG) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) SPEARMINT OIL (UNII: C3M81465G5) CARVONE, (+/-)- (UNII: 75GK9XIA8I) TRIISOSTEARIN (UNII: 71503RH8KG) TOCOPHEROL (UNII: R0ZB2556P8) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERIN (UNII: PDC6A3C0OX) CITRAL (UNII: T7EU0O9VPP) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) SHEA BUTTER (UNII: K49155WL9Y) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) ALLANTOIN (UNII: 344S277G0Z) GREEN TEA LEAF (UNII: W2ZU1RY8B0) JOJOBA OIL (UNII: 724GKU717M) CARNAUBA WAX (UNII: R12CBM0EIZ) SQUALANE (UNII: GW89575KF9) VITIS VINIFERA SEED (UNII: C34U15ICXA) Product Characteristics Color Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65278-105-01 14 g in 1 TUBE; Type 0: Not a Combination Product 12/09/2012 2 NDC:65278-105-02 14 g in 1 CARTON; Type 0: Not a Combination Product 12/09/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/09/2012 Labeler - June Jacobs Labs, LLC (082439410) Establishment Name Address ID/FEI Business Operations June Jacobs Labs, LLC 122610681 manufacture(65278-105)