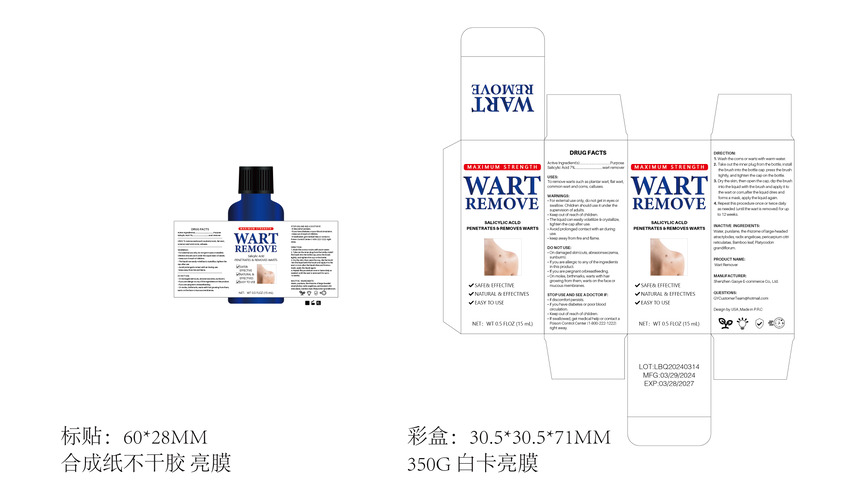
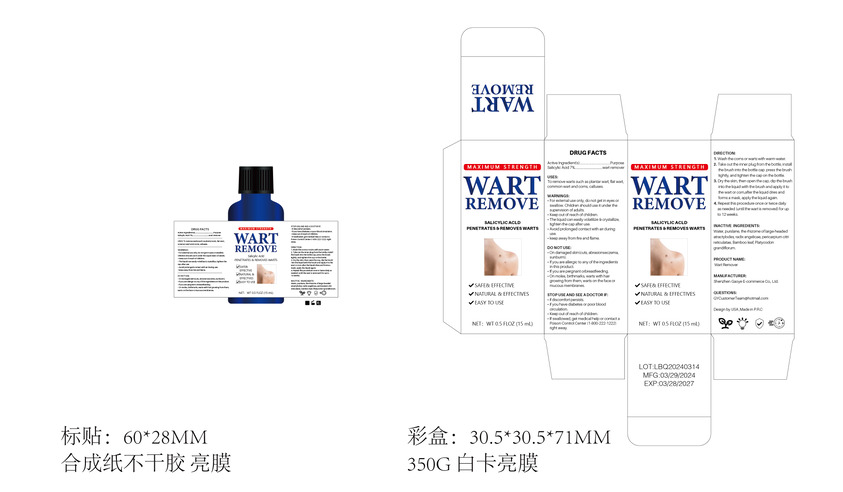
Label: WART REMOVE liquid
- NDC Code(s): 84322-002-01
- Packager: Shenzhen Gaoye E-commerce Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
1. Wash the corns or warts with warm water.
2. Take out the inner plug from the bottle, install the brush into the bottle cap. press the brush
tightly, and tighten the cap on the bottle.
3. Dry the skin, then open the cap, dip the brush into the liquid with the brush and apply it to
the wart or corn,after the liquid dries and forms a mask, apply the liquid again.
4. Repeat this procedure once or twice daily as needed (until the wart is removed) for up
to 12 weeks. - Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WART REMOVE
wart remove liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84322-002 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 7 g in 100 mL Inactive Ingredients Ingredient Name Strength ANGELICA DAHURICA VAR. FORMOSANA WHOLE (UNII: 247A107296) PURSLANE (UNII: M6S840WXG5) BAMBUSA VULGARIS LEAF (UNII: EMY54R518C) WATER (UNII: 059QF0KO0R) TANGERETIN (UNII: I4TLA1DLX6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84322-002-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 05/13/2024 Labeler - Shenzhen Gaoye E-commerce Co., Ltd (412499698) Establishment Name Address ID/FEI Business Operations Shenzhen Gaoye E-commerce Co., Ltd 412499698 manufacture(84322-002)