Label: LIDOTRAL TWO PERCENT- lidocaine hydrochloride spray
- NDC Code(s): 59088-304-03
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Lidotral® 2% Spray contains 20 mg of Lidocaine HCl per gram in a vehicle of Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aminomethyl Propanol, Aqua (Purified Water), Benzyl Alcohol, Ethyl Alcohol, PEG-8, Rosmarinus Officinalis (Rosemary) Leaf Oil, Fragrance.
Lidocaine HCI is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl), and has the following structure: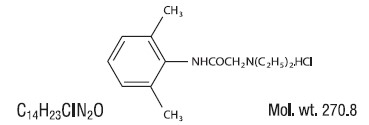
Lidotral® 2% Spray is a combination of ingredients with analgesics and anesthetic properties used in a unique way to maximize its pain-relieving effects to aid in addition to other therapies. This offers long lasting relief for a variety of pain conditions.
- INDICATIONS
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
Lidocaine is a common local anesthetic that relieves itching, burning, and pain. Topically, it blocks both initiation and conduction or nerve impulses by decreasing ionic flux through the neuronal membrane. Since it penetrates the skin, it creates an anesthetic effect by not just preventing pain signals from propagating to the brain out by stopping them before they begin.
Pharmacokinetics
Absorption:
Lidocaine- Lidocaine may be absorbed following topical administration to mucous membranes, its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation in the liver. Lidocaine is metabolized rapidly by the liver and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexylidide. The pharmacological/ toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline. The plasma binding of lidocaine is dependent on drug concentration and the fraction bound decreases with increasing concentration. At concentrations of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid glycoprotein. Lidocaine crosses the blood-brain and placental barriers, presumably by passive diffusion. Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics, but may increase the accumulation of metabolites. Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey, arterial blood levels of 18-21 g/mL have been shown to be threshold for convulsive activity.
Excretion:
Lidocaine- Lidocaine and its metabolites are excreted by the kidneys. Less than 10% of lidocaine is excreted unchanged. The half-life of lidocaine elimination from the plasma following IV administration is 81 to 149 minutes (mean 107 ± 22 SD, n = 15). The systemic clearance is 0.33 to 0.90 L/min (mean 0.64 ± 0.18 SD, n = 15). - CONTRAINDICATIONS
-
WARNINGS
For external use only.Not for ophthalmic use.
EXCESSIVE DOSING
Excessive dosing by applying Lidotral® 2% Spray to larger areas or for longer than the recommended wearing time could result in increased absorption of lidocaine and high blood concentrations, leading to serious adverse effects (see ADVERSE REACTIONS, Systemic Reactions). Lidocaine toxicity could be expected at lidocaine blood concentrations above 5 mcg/mL. The blood concentration of lidocaine is determined by the rate of systemic absorption and elimination. Longer duration of application, application of more than the recommended number of patches, smaller patients, or impaired elimination may all contribute to increasing the blood concentration of lidocaine. With recommended dosing, the average peak blood concentration is about 0.13 mcg/ mL, but concentrations higher than 0.25 mcg/mL have been observed in some individuals.
-
PRECAUTIONS
Allergic Reactions:Patients allergic to para-aminobenzoic acid derivatives (procaine, tetracaine, benzocaine, etc.) have not shown cross sensitivity to lidocaine. However, Lidotral® 2% Spray should be used with caution in patients with a history of drug sensitivities, especially if the etiologic agent is uncertain.
Non-intact Skin: Application to broken or inflamed skin, although not tested, may result in higher blood concentrations of lidocaine from increased absorption. Lidotral® 2% Spray is only recommended for use on intact skin.
External Heat Sources: Placement of external heat sources, such as heating pads or electric blankets, over Lidotral® 2% Spray is not recommended as this has not been evaluated and may increase plasma lidocaine levels.
Eye Exposure: The contact of Lidotral® 2% Spray with eyes, although not studied, should be avoided based on thefindings of severe eye irritation with the use of similar products in animals. If eye contact occurs, immediately wash out the eye with water or saline and protect the eye until sensation returns.
-
Drug Interactions
Antiarrhythmic Drugs: Lidotral® 2% Spray should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic.
Local Anesthetics: When Lidotral® 2% Spray is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered. -
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: A minor metabolite, 2,6-xylidine, has been found to be carcinogenic in rats. The blood concentration of this metabolite is negligible following application of lidocaine.
Mutagenesis: Lidocaine HCl is not mutagenic in Salmonella/mammalian microsome test nor clastogenic in chromosome aberration assay with human lymphocytes and mouse micronucleus test.
Impairment of Fertility: The effect of lidocaine on fertility has not been studied. -
Pregnancy
Teratogenic Effects: Pregnancy Category B. Lidocaine has not been studied in pregnancy. Reproduction studies with lidocaine have been performed in rats at doses up to 30 mg/kg subcutaneously and have revealed no evidence of harm to the fetus due to lidocaine. There are, however, no adequate and well- controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, lidocaine should be used during pregnancy only if clearly needed.
Labor and Delivery
Lidocaine has not been studied in labor and delivery. Lidocaine is not contraindicated in labor and delivery. Should lidocaine be used concomitantly with other products containing lidocaine, total doses contributed by all formulations must be considered. - Nursing Mothers
- Pediatric Use
-
ADVERSE REACTIONS
ADVERSE REACTIONS
Application Site Reactions
Even though adverse reactions are rare, a very small percentage of patients experience an unpleasant burning sensation, redness, warmth, or stinging. It is advisable to apply a small amount on the forearm prior to first use. If any of these effects persists or worsens, contact your physician or pharmacist immediately. This medication is not absorbed systemically but if any serious side effects (i.e. rash, itching/swelling, severe dizziness) are experienced, discontinue use immediately and contact your pharmacist or physician. This is not a complete list of all side effects that may occur. You may report side effects to the FDA at 800-FDA-1088 or at http://www.fda.gov/medwatch.
Allergic Reactions
Allergic and anaphylactoid reactions associated with lidocaine, although rare, can occur. They are characterized by angioedema, bronchospasm, dermatitis, dyspnea, hypersensitivity, laryngospasm, pruritus, shock, and urticaria. If they occur, they should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Other Adverse Events
Due to the nature and limitation of spontaneous reports in post marketing surveillance, causality has not been established for additional reported adverse events including: Asthenia, confusion, disorientation, dizziness, headache, hyperesthesia, hypoesthesia, lightheadedness, metallic taste, nausea, nervousness, pain exacerbated, paresthesia, somnolence, taste alteration, vomiting, visual disturbances such as blurred vision, flushing, tinnitus, and tremor.
Systemic (Dose-Related) Reactions
Systemic adverse reactions following appropriate use of lidocaine is unlikely, due to the small dose absorbed (see CLINICAL PHARMACOLOGY, Pharmacokinetics). Systemic adverse effects of lidocaine are similar in nature to those observed with other amide local anesthetic agents, including CNS excitation and/or depression (light-headedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest). Excitatory CNS reactions may be brief or not occur at all, in which case the first manifestation may be drowsiness merging into unconsciousness. Cardiovascular manifestations may include bradycardia, hypotension and cardiovascular collapse leading to arrest.
To report SUSPECTED ADVERSE REACTIONS, contact Actavis at 1-800- 272-5525 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch for voluntary reporting of adverse reactions. -
OVERDOSAGE
Lidocaine overdose from cutaneous absorption is rare, but could occur. If there is any suspicion of lidocaine overdose (see ADVERSE REACTIONS, Systemic Reactions), drug blood concentration should be checked. The management of overdose includes close monitoring, supportive care, and symptomatic treatment. Dialysis is of negligible value in the treatment of acute overdose with lidocaine.
In the absence of massive topical overdose or oral ingestion, evaluation of symptoms of toxicity should include consideration of other etiologies for the clinical effects, or over dosage from other sources of lidocaine or other local anesthetics.
The oral LD50 of lidocaine HCl is 459 (346 to 773) mg/kg (as the salt) in non-fasted female rats and 214 (159 to 324) mg/kg (as the salt) in fasted female rats, which are equivalent to roughly 4000 mg and 2000 mg, respectively, in a 60 to 70 kg man based on the equivalent surface area dosage conversion factors between species. -
DOSAGE AND ADMINISTRATION
Adults & children 4 years of age and older: spray to the affected area(s) two or three times daily or as directed by alicensed healthcare practitioner.
Children under 4 years of age: consult a licensed healthcare practitioner.
Apply Lidotral® 2% Spray to intact skin to cover the most painful area. Smaller areas of treatment are recommended in a debilitated patient, or a patient with impaired elimination.
When Lidotral® 2% Spray is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all formulations must be considered. - HOW SUPPLIED
- Lidotral® 2% Spray
-
INGREDIENTS AND APPEARANCE
LIDOTRAL TWO PERCENT
lidocaine hydrochloride sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-304 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) ROSEMARY OIL (UNII: 8LGU7VM393) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) BENZYL ALCOHOL (UNII: LKG8494WBH) ALCOHOL (UNII: 3K9958V90M) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-304-03 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 05/14/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/14/2024 Labeler - PureTek Corporation (785961046)


