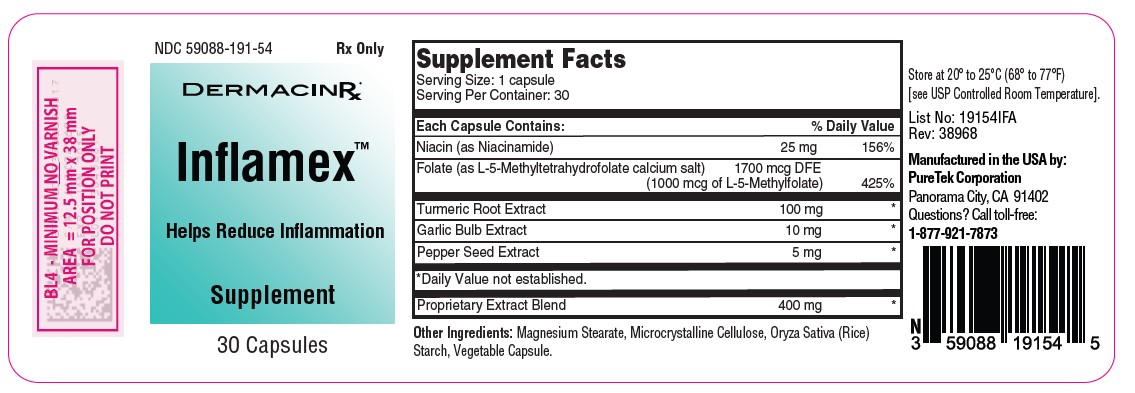
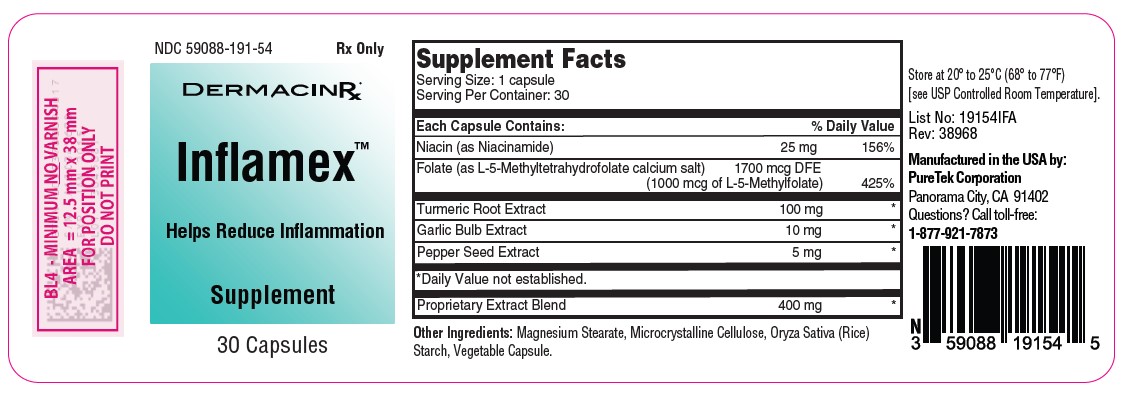
Label: INFLAMEX- multivitamin capsule
- NDC Code(s): 59088-191-54
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
lnflamex™ is a unique formulation of selected natural ingredients such as Turmeric, Black Pepper, Garlic and Proprietary Extract Blend combined with Niacin and Folate. This potent blend is designed to support your body's response to inflammation and its natural healing processes.
Each capsule contains:
Niacin (as Niacinamide) ....……………………………….…......25 mg
Folate (as L-5-Methyltetrahydrofolate calcium salt) ..........1700 mcg DFE
(1000 mcg of L-5-Methylfolate)
Turmeric Root Extract……………………………….…………100 mg
Garlic Bulb Extract……………………………….…………….. 10 mg
Pepper Seed Extract……………………………….…………….. 5 mg
Proprietary Extract Blend ………………………….…………. 400 mg - Other Ingredients:
-
Contraindications:
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. lnflamex™ is contraindicated in patients with gastrointestinal conditions, such as gastric ulcers or gastroesophageal reflux disease (GERD), as they may experience irritation or worsening of symptoms due to the presence of ingredients like garlic and black pepper. Patients with blood clotting disorders or who are on anticoagulant therapy should use caution when consuming high doses of ingredients like garlic, turmeric, and black pepper, as they may have mild blood-thinning effects.
-
Warnings:
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor. Administration of folate alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
Precautions:
Folate in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folate to patients with undiagnosed anemia since folate may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. The patient's medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
-
Pregnancy and Lactation:
Not recommended for use during pregnancy or lactation without consulting with a healthcare professional.
For use on the order of a licensed healthcare practitioner.
If a patient experiences any side effects, call your doctor. To report side effects, call PureTek Corporation at 1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. -
Adverse Reactions:
Allergic reactions to any of the ingredients can occur, ranging from mild skin irritation to severe anaphylaxis in rare cases. Allergic sensitization has been reported following both oral and parenteral administration of folate.
Niacinamide, a form of niacin, may cause flushing, itching, and tingling sensations in some individuals, particularly when taken in higher doses. This effect is usually harmless but can be uncomfortable. Some individuals may experience gastrointestinal symptoms such as nausea, bloating, or diarrhea, especially when consuming higher doses of certain ingredients like garlic and black pepper. Certain ingredients in the product, such as garlic and black pepper, may interact with medications. Garlic may potentiate the effects of anticoagulant medications, while black pepper may affect the metabolism of certain drugs. - Dosage and Administration:
- How Supplied:
- Storage:
- Inflamex™
-
INGREDIENTS AND APPEARANCE
INFLAMEX
multivitamin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-191 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK PEPPER (UNII: KM66971LVF) (BLACK PEPPER - UNII:KM66971LVF) BLACK PEPPER 5 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 25 mg GARLIC (UNII: V1V998DC17) (GARLIC - UNII:V1V998DC17) GARLIC 10 mg TURMERIC (UNII: 856YO1Z64F) (TURMERIC - UNII:856YO1Z64F) TURMERIC 100 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 1700 ug Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) GELLAN GUM (LOW ACYL) (UNII: 7593U09I4D) STARCH, RICE (UNII: 4DGK8B7I3S) HYPROMELLOSES (UNII: 3NXW29V3WO) Product Characteristics Color orange (Light orange with black specks) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-191-54 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/12/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/12/2024 Labeler - PureTek Corporation (785961046)